Epidemiology and risk factors
Pneumocystis jirovecii (formerly Pneumocystis carinii ) is an infrequent though potentially deadly cause of fungal pneumonia ( P. jirovecii pneumonia [PJP]) in immunocompromised patients. P. jirovecii is a ubiquitous unicellular organism found around the world with an affinity for the respiratory tract. Most individuals are exposed in childhood and many are colonized, but disease occurs almost exclusively in immunocompromised hosts. Individuals with impaired cellular immunity, which is common in the setting of malignancy, primary immunodeficiencies, or a history of solid organ or bone marrow transplant, are vulnerable and repeat exposure can lead to fatal infection if untreated.
Aside from human immunodeficiency virus (HIV), the most robust studies of PJP have been in hematopoietic stem cell transplant (HSCT) patients. PJP was a common cause of nonbacterial pneumonia and was associated with substantial morbidity and mortality before the implementation of prophylaxis with trimethoprim-sulfamethoxazole (TMP-SMX) in the late 1970s. PJP infections in HSCT patients are no longer common (incidence of 0.63% for allogeneic and 0.28% for autologous transplants), although infections are still associated with high mortality in breakthrough cases with a 6.9-fold increased risk of death. HSCT patients at highest risk are those with graft-versus-host disease and poor immune reconstitution.
In patients with hematologic malignancy, PJP risk is related to the underlying disease as well as the treatment regimen. The most significant risk factors include acute lymphoblastic leukemia, corticosteroids (particularly 2 mg/kg per day or more of prednisone or equivalent), and use of T-cell–depleting agents, such as alemtuzumab. High-intensity chemotherapy and prolonged CD4 lymphopenia also appear to be associated with an increased risk of PJP. The risk of PJP in children with acute myelogenous leukemias and solid tumors is not well defined, with a wide range of incidences reported (0.5 to 25 %) in children with solid tumors. Steroid use is a key factor in determining PJP risk in these populations.
Reported rates of PJP in patients receiving solid organ transplants (SOTs) are between 5% and 15%. The risk correlates with increased immunosuppression, temporal proximity to transplant, corticosteroid use, therapies to treat rejection, as well as low CD4 counts, and lymphopenia.
Clinical manifestations
Classically, Pneumocystis pneumonia has an acute presentation in non-HIV immunocompromised individuals with symptoms of fever, cough, and dyspnea evolving over a few days, although symptoms can progress more gradually over the course of 1 to 2 weeks. Tachypnea, tachycardia, and evidence of increased work of breathing, with nasal flaring and intercostal retractions, are common in children, whereas lung auscultation is often normal. Illness can progress rapidly, and many children require intensive care unit admission and mechanical ventilation. As the clinical presentation is nonspecific, it is critical to maintain a high index of suspicion in at-risk patients to facilitate early diagnosis and treatment. Co-infection with other pathogens, including cytomegalovirus as well as bacteria, fungi, and respiratory viruses, occurs frequently in patients with PJP and may influence the clinical presentation. ,
Impaired oxygenation is common in PJP and can be severe. The degree of hypoxemia at presentation, as measured by the arterial blood oxygen tension (Pao 2 ), is commonly used to evaluate disease severity. Contrary to conventional teaching, PJP may present without hypoxemia in non-HIV immunocompromised individuals. A recent small retrospective study in adult SOT recipients found that less than half of SOT patients with PJP were hypoxemic on admission. However, the lack of hypoxemia could reflect earlier diagnosis and therefore less severe disease, because of the high index of suspicion for PJP in the SOT population, rather than an actual difference in how the disease presents.
Imaging
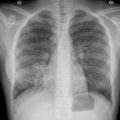
Chest radiographic findings in PJP are nonspecific, most commonly showing bilateral perihilar interstitial infiltrates that spread peripherally. Chest radiographic findings can be normal early in the disease. , , High-resolution computed tomography is much more sensitive than chest radiography and typically shows bilateral patchy ground-glass opacities, concentrated in the perihilar regions, with or without consolidation. Cystic lesions and nodules can also be seen, although less commonly. Patients receiving inhaled pentamidine prophylaxis more commonly have upper lobe infiltrates, likely because of decreased deposition of pentamidine in this area. Pneumothorax is a recognized complication of PJP and may be more common in HSCT patients.
Disease prophylaxis and prevention
Administration of PJP prophylaxis significantly reduces the risk of PJP in high-risk individuals. The generally accepted threshold for starting prophylaxis is greater than 3.5% risk of developing PCP to justify the potential toxicities. Indications for PJP prophylaxis in pediatric oncology and transplant populations are listed in Table 31.1 and suggested dosing in Table 31.2 .
| Condition/Indication | Duration/Notes |
| Oncology | |
| Acute lymphoblastic leukemia Acute myeloid leukemia | Continue from induction to end of maintenance |
| Solid tumors | When treatment is likely to result in lymphopenia for the duration of chemotherapy |
| Hematopoietic Stem Cell Transplant | |
| Allogeneic | Continue from engraftment through at least 6 months and until discontinuation of immunosuppression |
| Autologous | Generally given for 3-6 months after transplant |
| Solid Organ Transplant a | |
| Heart, liver, kidney | 6-12 months after transplant |
| Lung, small bowel | Often lifelong |
| Treatment of rejection | For 3-6 months after treatment (depending on treatment) b |
| Other | |
| Steroids (>0.4 mg/kg per day or >16 mg/day for >1 month) | For the duration of therapy |
| Rituximab Alemtuzumab | For ≥6 months after treatment |
a Some experts recommend lifelong prophylaxis for all solid organ transplant recipients.
b Prophylaxis for 3 months with pulse steroids, 6 months with antithymocyte globulin.
| Agent | Dose and Frequency | |
| Trimethoprim-sulfamethoxazole | 5-10 mg/kg per day of trimethoprim component divided into twice daily dosing. Given every day or 2-3 days per week. | Maximum dose of trimethoprim 320 mg/day |
| Dapsone | 2 mg/kg once daily if >1 month of age | Maximum 100 mg daily |
| Atovaquone | 30 mg/kg once daily if >2 years 45 mg/kg once daily if 4 months to 2 years | Maximum 1500 mg daily |
| Pentamadine | 9 mg/kg every 4 weeks (age <5 years) | Maximum 300 mg/day |
| aerosolized | 300 mg every 4 weeks (age >5 years) | |
| Intravenously | 4 mg/kg every 2-4 weeks (age >2 years) |
Trimethoprim-sulfamethoxazole
TMP-SMX is the most studied agent for PJP prophylaxis and is considered the agent of choice to prevent disease. A 2014 Cochrane meta-analysis evaluating the effectiveness of TMP-SMX for prophylaxis of PJP in hematologic malignancy, stem cell transplant, and SOT patients found that the risk of PJP was 85% lower in patients receiving TMP-SMX compared with those receiving no PJP prophylaxis. Multiple guidelines, including those of the American Society for Blood and Marrow Transplantation, the American Transplant Society, the American Society of Clinical Oncology, and the Infectious Diseases Society of America, support the use of TMP-SMX as the first-line agent for PJP prophylaxis. In addition to its activity against Pneumocystis, TMP-SMX is protective against toxoplasmosis, listeriosis, and nocardiosis if given at sufficient doses.
Side effects of TMP-SMX include rash, fever, neutropenia, pancytopenia, hepatitis, and anaphylaxis. The Cochrane review of TMP-SMX prophylaxis in non-HIV immunocompromised patients reported that adverse events leading to permanent discontinuation of TMP-SMX occurred in approximately 3.1% of adult patients, but no serious adverse events were reported in children. TMP-SMX should be used with caution in patients who are glucose 6-phophate dehydrogenase (G6PD) deficient as hemolysis may occur. Mutations leading to resistance to TMP-SMX have been reported, though fortunately, they have been uncommon.
Multiple TMP-SMX prophylactic dosing regimens have been used and for PJP, and there is no clear difference in efficacy between twice-weekly and other more frequent dosing schedules. There may be beneficial effects of more frequent dosing for prophylaxis against non-PJP infections in some high-risk populations. TMP-SMX needs to be dose adjusted in patients with creatinine clearance less than 30 mL/min.
Alternative agents
Alternatives to TMP-SMX include intravenous (IV) and inhaled pentamidine, dapsone, and atovaquone; however, there have been few prospective comparison studies and thus there is no clear consensus with respect to the preferred alternative agent.
Pentamidine is the most studied second-line prophylaxis option. A retrospective study in adult HSCT patients found that patients receiving inhaled pentamidine had a higher probability of developing PJP (9.1%) than those receiving TMP-SMX (0%). There is some concern that the IV form of pentamidine may not result in sufficient intra-alveolar concentrations to be protective ; however, recent retrospective studies in pediatric oncology, HSCT and SOT patients report good outcomes with inhaled and IV pentamidine. , The dosing interval for pentamidine is traditionally every 4 weeks, although a higher frequency has been used in young children, particularly those younger than 2 years, safely. Pentamidine does not have activity against toxoplasmosis.
The inhaled form of pentamidine can be associated with coughing and wheezing that can be reduced with pretreatment with β-adrenergic agonists. Bronchospasm may be more common or severe in patients with chronic respiratory conditions. Inhaled pentamidine therapy requires a specific nebulizer capable of producing particles smaller than 1 μm, as larger particles may be unable to reach the alveoli and would be less effective. The use of inhaled pentamidine in young children may be limited by their ability to coordinate and cooperate with medication inhalation. There have been reports of extrapulmonary infections with Pneumocystis in patients using inhaled pentamidine. The American Society of Transplantation recommends that inhaled pentamidine be considered a third-line agent, as there are more breakthrough infections than with atovaquone and dapsone.
Atovaquone is another second-line agent that is available only in oral solution form. In HIV-infected individuals, atovaquone has been compared with once-daily dapsone and aerosolized monthly pentamidine with no significant differences in mortality or PJP infections, , but data for non-HIV-immunocompromised children are scarce. A small prospective study comparing atovaquone and TMP-SMX in adult autologous transplant patients did not identify any cases of PJP with either drug but found that TMP-SMX was discontinued significantly more frequently because of intolerance. Atovaquone is generally well tolerated with minimal side effects, including rash, gastrointestinal symptoms, and headache. It also has the advantage of having activity against toxoplasma. High cost and poor palatability are the most common limitations in the use of atovaquone. There have also been reports of development of PJP resistance in a transplant center with widespread use.
The final prophylactic option is dapsone. There has been limited study of dapsone prophylaxis outside the HIV-infected population, where dapsone appears to have efficacy similar to atovaquone in patients who could not tolerate TMP-SMX. A retrospective study in HSCT recipients who could not tolerate TMP-SMX reported a 7.2% incidence of PJP in the dapsone group, using 3 times weekly dosing, compared with 0.37% in the TMP-SMX group ( P < .001). The same group reported a 1.3% incidence of PJP in HSCT recipients taking daily dapsone prophylaxis, suggesting that daily prophylaxis may be more effective.
Dapsone can induce neutropenia, rash, and gastrointestinal upset. It can also cause methemoglobinemia. It should be avoided in those with severe TMP-SMX reactions as there may be cross-reactivity between the two drugs. Children should be screened for G6PD deficiency before initiation of dapsone treatment, as it can cause hemolytic anemia in children with G6PD deficiency and should be avoided in this population. Dapsone may have some activity against toxoplasma but is not recommended as monotherapy for toxoplasma prophylaxis.
Duration of prophylaxis
PJP prophylaxis should be continued throughout the periods of most intense immunosuppression, but the optimal duration of PJP prophylaxis is unknown. In SOT recipients, PJP prophylaxis is generally provided for at least 6 to 12 months after transplant. Some experts recommend lifelong prophylaxis for all SOT recipients. PJP prophylaxis should be extended or restarted in patients receiving high-dose steroids or treatments for rejection. Given their intense immunosuppression, lifelong prophylaxis is often provided for lung and small bowel transplant recipients. Lifelong secondary prophylaxis is recommended in patients with a history of PJP.
In HSCT patients, TMP-SMX is usually held until engraftment because of concern for potential marrow suppression, although it is unclear if this is necessary. Prophylaxis should continue for at least 6 months after allogeneic transplant. Longer prophylaxis is required for those with graft-versus-host-disease and/or ongoing immunosuppression. In autologous transplants, prophylaxis is often provided for 3 to 6 months after transplant. Although good-quality evidence for PJP prophylaxis in children with solid tumors and acute myeloid leukemia is lacking, it is common practice to prescribe prophylaxis throughout the course of chemotherapy.
Diagnosis
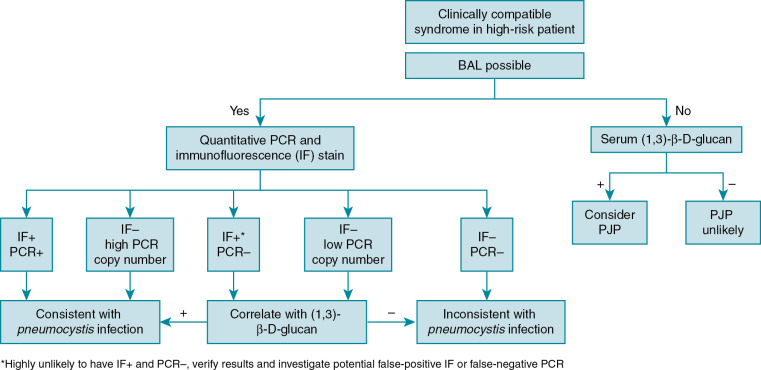
PJP diagnosis can be quite difficult because of wide variations in presentation. Even when suspected, the diagnosis can be difficult to confirm as P. jirovecii cannot be cultured through traditional methods. Bronchoalveolar (BAL) fluid is the preferred specimen to diagnose PJP. If BAL fluid is not available, multiple induced sputum samples are often required to establish the diagnosis, as there are fewer cysts in the upper respiratory airways. Specific staining techniques of respiratory specimens, including Giemsa, can be used establish the diagnosis and to identify trophic forms, and toluidine blue or calcofluor white can be used to detect cysts. Immunofluorescent monoclonal antibodies to PJP that can identify both trophic forms and cysts are commonly used to establish the diagnosis. Staining and immunofluorescent assays may have sensitivities of 90% or better in BAL specimens, but sensitivity is lower in sputum specimens.
Polymerase chain reaction (PCR) is the most sensitive method to establish the diagnosis of PJP; however, PCR diagnosis is complicated by the possibility of detecting Penumocystis colonization in the absence of clinical disease. There are a variety of PJP PCR assays with differing targets and performance characteristics. Quantitative PJP PCR assays appear to be more promising than qualitative assays, but there is no currently established threshold to distinguish colonization from clinical PJP disease.
Serum (1,3)-β-D-glucan can be used as a screening tool for PJP, especially when bronchoscopy is not feasible or immediately available. A negative (1,3)-β-D-glucan test result has a high negative predictive value and can be used to exclude PJP. It should not be used in isolation to diagnose PJP as it is a nonspecific marker positive in many fungal infections, including infections with Candida and Aspergillus species. Lactate dehydrogenase is commonly obtained but is not particularly helpful because of its low sensitivity and specificity.
Diagnostic algorithms using multiple diagnostic methods are recommended to establish the diagnosis of PJP , ( Fig. 31.1 ).