Platelet transfusion therapy has become an integral part of the treatment of patients with hematological and solid tumor malignancy receiving chemotherapy. Since its introduction almost 60 years ago, several advances and refinements have been introduced in the collection, storage, and administration to improve the safety and efficacy of platelet transfusion. This review summarizes the current practice and clinical approach to patients with thrombocytopenia. Existing evidence-based guidelines for appropriate platelet transfusion is reviewed.
Key points
- •
In the United States, platelets are collected in citrate anticoagulants from single units or by apheresis procedure.
- •
To prevent bleeding in thrombocytopenic patients, it is a common practice to transfuse platelets when platelet counts reach a trigger threshold (prophylactic transfusion).
- •
The transfusion of all blood products including platelets can cause viral infection, such as hepatitis B, C, West Nile virus, and HIV, although the modern nucleic acid–based testing has reduced the risk to a very low level.
- •
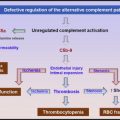
Platelet transfusions are considered risky in patients with thrombotic thrombocytopenic purpura because it can exacerbate the microvascular thrombosis.
- •
Because treatment of alloimmune refractory thrombocytopenia is difficult, costly, and often ineffective, it is critical to prevent alloimmunization.
Historical perspective
Duke reported the earliest platelet transfusion in a landmark article at the beginning of the last century in 3 patients with idiopathic thrombocytopenic purpura. He achieved temporary cessation of bleeding and an increase in platelet count in 2 patients by transfusing directly the unmodified whole blood. These earlier attempts were limited by lack of platelet concentrates and in vitro activation of platelets. The modern era of platelet transfusion started with demonstration by Gaydos and colleagues that prophylactic transfusions of platelets in patients receiving chemotherapy dramatically reduced the incidence of fatal bleeding complications and allowed completion of chemotherapy. They also pioneered apheresis techniques in harvesting of platelets for transfusion. The introduction of storage at room temperature, the use of gas-permeable containers, agitation during storage, and the use of acid citrate as the anticoagulant led to better platelet preservation and increased storage time, allowing widespread availability. Platelets have become the second commonest blood components transfused.
Historical perspective
Duke reported the earliest platelet transfusion in a landmark article at the beginning of the last century in 3 patients with idiopathic thrombocytopenic purpura. He achieved temporary cessation of bleeding and an increase in platelet count in 2 patients by transfusing directly the unmodified whole blood. These earlier attempts were limited by lack of platelet concentrates and in vitro activation of platelets. The modern era of platelet transfusion started with demonstration by Gaydos and colleagues that prophylactic transfusions of platelets in patients receiving chemotherapy dramatically reduced the incidence of fatal bleeding complications and allowed completion of chemotherapy. They also pioneered apheresis techniques in harvesting of platelets for transfusion. The introduction of storage at room temperature, the use of gas-permeable containers, agitation during storage, and the use of acid citrate as the anticoagulant led to better platelet preservation and increased storage time, allowing widespread availability. Platelets have become the second commonest blood components transfused.
Platelet life span
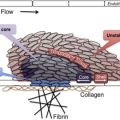
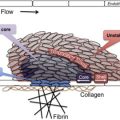
Circulating platelets have a life span of 10 days and about 10% of platelets are removed every day mainly via the spleen and the liver. What determines the end of platelet life span in the circulation is not known and there are several hypotheses. Phosphatidylserine, a well-known macrophage recognition signal for clearance of apoptotic cells, could play a role. In resting platelets, phosphatidylserine is located on the inner leaflet of the membrane bilayer and following platelet activation there is trans-bilayer movement from the inner to the outer leaflet. Both an activation-dependent and a senescence-induced pathway leading to a trans-bilayer movement of phosphatidylserine have been described. According to “the multiple-hit model,” the life span of platelets is determined by the damages to (or activations of) platelets in the blood circulation. Platelet clearance by macrophages depends on the number of “hits” accumulated during platelet life span in the circulation. Logistic regression analysis of the disappearance curves of labeled transfused platelets by best fitting models suggests a complicated clearance process involving linear (due to senescence) and random (due to activation) components. In recent years, an alternate model, based on forward genetic studies with N -ethyl- N -nitrosourea–induced mutagenesis in mice, suggests platelets are formed with an “internal clock” and their survival is determined by intrinsic mechanisms rather than external hits. Platelets express the Bcl-2 family of proteins Bax and Bak in the mitochondria. These proteins govern mitochondrial outer membrane integrity and can be either pro-apoptotic (Bax, BAD, and Bak among others) or anti-apoptotic (Bcl-x). Knock-out of antiapoptotic Bcl-x(L) reduces platelet half-life and causes thrombocytopenia. Deletion of proapoptotic Bak corrects these defects, and platelets from Bak-deficient mice circulate for a longer time than normal platelets. Apoptotic stimuli are thought to activate BH3-domain–containing members of this protein family to initiate Bax/Bak-dependent apoptosis. ABT737, a synthetic BH3 mimetic reagent, induces the mitochondrial pathway of apoptosis by binding to Bcl-2 and Bcl-XL and blocking their inhibitory effect on the proapoptotic Bax and Bak. ABT737 has been used to mimic in vivo senescence and phosphatidylserine exposure in platelets.
There is evidence for a fixed daily requirement of platelets. A large number of studies have shown that platelets support the function of the vascular endothelium, and in animals thrombocytopenia has been shown to increase protein permeability across the endothelium of the lung, ear, thyroid, and heart, which can be reversed by infusion of platelet-rich plasma. These data are also consistent with clinical observations that recovery of transfused platelets is decreased in thrombocytopenia and may account for the spontaneous unprovoked bleeding seen in severe thrombocytopenia. Daily obligatory requirement is estimated to be about 7100/μL, about 18% of daily turnover.
The life span of platelets stored at room temperature is remarkably similar to the life span of platelets in the circulation. Platelet recovery after transfusion decreases by 10% for each day of storage outside of the body, which is very close to what is expected from the in vivo life span of platelets. The changes in stored platelets are collectively known as platelet storage lesions. These changes result not only in reduced response to agonists but also in an accelerated clearance from the circulation following transfusion. Increased phosphatidylserine expression and caspase-3 activity in stored platelets has been observed and phosphatidylserine-expressing platelets are rapidly cleared from the circulation.
Platelets that are stored at 4°C rapidly undergo shape change and lose responsiveness to agonists, and the in vivo recovery of cold-stored platelets is poor. The mechanism for the rapid clearance of cold-stored platelets is not known and it was postulated that clustering of the GPIb/V/IX complex on the surface of platelets results in platelet clearance by α M β 2 integrin on hepatic macrophages that recognizes the β- N -acetylglucosamine (βGlcNAc)–terminating immature glycans. Masking the penultimate βGlcNAc by galactosylation by uridine diphosphate galactose-galactose prevented phagocytosis of cold-stored murine platelets. However, in a human phase I study, galactosylation with uridine diphosphate galactose-galactose did not prevent the rapid clearance of cold-stored platelets and the clinical relevance of these findings in transfusion medicine is not clear.
Platelet collection and processing
Single Donor Platelets
In the United States, platelets are collected in citrate anticoagulants from single units or by apheresis procedure. Single-donor blood is collected in citrate anticoagulant containing adenine in a pH of 5.3 to 5.9. The platelet-rich plasma is obtained by centrifugation at room temperature at 2100 g for 2.7 minutes (soft spin). The platelet-rich plasma is again centrifuged at a higher speed (hard spin); the supernatant is transferred in a closed system to another container leaving approximately 40 to 70 mL of plasma with the platelet suspension, and it is stored at room temperature with constant agitation. The typical yield is 5.0 × 10 10 platelets per unit.
Apheresis Units
Increasingly, platelets are harvested by the apheresis method. Apheresis platelets are especially useful in collecting from family members, HLA-compatible donors, or individuals with rare phenotype. Apheresis has the advantage of collecting from a single donor that limits donor exposure and provides platelets with consistent quality. The apheresis donor unit contains at least 3 × 10 11 platelets per unit. More than 60% of platelets infused in the United States are from apheresis donors. Donors can donate twice a week. The newer automated apheresis instruments are capable of collecting single, double, and even triple apheresis units from a donor and they can be programed to yield optimal amount based on donor hematocrit, height, and weight.
Buffy Coat Platelets
Platelets can also be obtained using the buffy coat method, by centrifuging the whole blood (hard spin), and removing the supernatant platelet-poor plasma and bottom red blood cells, leaving behind the buffy coat, which contains mostly platelets and white blood cells. The buffy coat is further centrifuged to remove white blood cells and red blood cells. Platelets prepared by the buffy coat method are less activated, and in addition to a slightly higher platelet yield, it is easier to institute leukocyte reduction and pathogen reduction technologies in this method. In some blood centers, excess plasma is replaced with a synthetic additive solution, which can prolong the storage time and reduce the amount of plasma transfused to patients. As a result, there is a reduction in the frequency of allergic reaction and possibly in transfusion-related acute lung injury. The buffy coat method and additive solutions are used in many blood centers in Canada and Europe.
Frozen Platelets
A considerable amount of work has been performed on freezing frozen platelets for clinical use. Frozen platelets can be stored for years and platelets with rare phenotypes can be saved for alloimmune thrombocytopenic patients. Typically, platelets are frozen in dimethyl sulfoxide as the cryopreservative and, after thawing, they are concentrated by centrifugation and the cryopreservative is removed and replaced with autologous plasma. Despite the demonstration of hemostatic effectiveness in vivo, the process is expensive and cumbersome with many logistical challenges.
Pathogen reduction techniques
Platelets are stored at room temperature and sepsis associated with bacterial proliferation is the major cause of morbidity and mortality associated with blood transfusion. The incidence of contamination has been reported between 1 in 1000 and 3000, whereas the risk of a clinically significant septic transfusion reaction was between 1 in 13,000 and 70,000 for single-donor platelets and platelets from whole blood, respectively. Because of this concern, the Food and Drug Administration allows storage of platelets at room temperature for only 5 days, and the American Association of Blood Banks requires a method to limit bacterial contamination. Platelet concentrates are tested for bacterial contamination using a growth-based system 24 hour after collection. This strategy detects only up to 40% of bacterial contamination. Consequently, several additional approaches have been under investigation to reduce the incidence of sepsis associated with platelet transfusions. Testing on the day of transfusion adds a measure of safety by detecting highly contaminated units. Platelet concentrates or apheresis units are now tested within 4 hours before transfusion. A currently available test based on the detection of lipoteichoic acid and lipopolysaccharide has a detection limit of 10 4 to 10 5 colony forming units per milliliter.
Another approach that is being actively tested is pathogen inactivation during storage, which is already implemented by certain countries. Psoralens and riboflavin along with ultraviolet (UV) radiation are being evaluated. Psoralens are compounds that intercalate into the helical regions of DNA and RNA. Upon UV radiation, adducts are formed between psoralen and the nucleic acids, rendering it incapable of replication. Amotosalen, a novel psoralen, has been shown to inactivate a variety of bacteria and viruses in platelet concentrates. UV radiation reduces amotosalen concentration to very low levels. In addition to Psoralens, other photosensitizers have been tried for pathogen inactivation. Methylene blue and riboflavin have been investigated for pathogen inactivation. Methylene blue plus visible light is known to induce DNA damage primarily at guanine bases. Riboflavin interacts with nucleic acids and enhances UV damage that results in direct electron transfer, production of singlet oxygen, generation of hydrogen peroxide, opening of purine ring, and DNA strand breakage. The advantage of riboflavin over methylene blue and psoralens is that riboflavin is an endogenous physiologic compound (vitamin B2). Recently, a novel procedure that uses short-wave UV light, without addition of any photoactive agent, has been developed.
In addition to inactivating bacteria and virus, pathogen inactivation offers several other advantages for platelet transfusion. The pathogen inactivation process will be effective in the prevention of cytomegalovirus infection, allowing more flexibility by omitting irradiation and testing for cytomegalovirus antibodies. This technology will also be effective against other transfusion-associated infection, such as malaria, and may allow the relaxation of donor deferral because of travel to endemic areas. Because infection is the major limitation for platelet storage, pathogen inactivation may allow extension of platelet storage beyond the current period of 5 days. Pathogen inactivation may also eliminate the current practice of bacterial detection before platelet transfusion. Inactivation of contaminating white blood cells will reduce the graft-versus-host disease and possibly alloimmunization. The disadvantages of the pathogen reduction process include added cost and the concerns about the long-term safety of potentially mutagenic agents used in this process.
Prophylactic and therapeutic transfusions
To prevent bleeding in thrombocytopenic patients, it is a common practice to transfuse platelets when platelet counts reach a trigger threshold (prophylactic transfusion) ( Fig. 1 ). Because most platelet transfusions are administrated to patients with hematologic malignancies, it is not a surprise that most of the studies on platelet transfusion are conducted in these patients. The risk of spontaneous bleeding in a patient with leukemia increases when platelet counts are less than 20,000/μL, and this number was considered to be the threshold for platelet transfusion in the 1960s. At that time, the antiplatelet effect of aspirin was not appreciated and aspirin was commonly prescribed to these patients for fever. Subsequent studies, when aspirin was no longer prescribed, revealed that a threshold of 10,000/μL could safely replace 20,000/μL in afebrile patients with no significant coagulopathy. The American Society of Hematology, the American Society Clinical Oncology, and the British Committee for Standards in Haematology (BCSH) have recommended a platelet count of 10,000/μL as a transfusion threshold. The same threshold is applicable to patients after hematopoietic stem cell transplantation or patients with solid tumors who developed thrombocytopenia during the course of chemotherapy, with the possible exception of patients with bladder tumors or necrotic tumors, who should probably receive prophylactic platelet transfusion with platelet counts less than 20,000/μL. Some studies have used successfully an even more restrictive threshold of 5000/μL in patients with acute leukemia in the absence of fever. In patients with acute promyelocytic leukemia the risk of bleeding is very high and the threshold of 20,000/μL is appropriate. In patients with chronic, severe, and stable thrombocytopenia, such as those with aplastic anemia or myelodysplastic syndrome, the recommendations for platelet transfusion are less clear. Most hematologists will not transfuse prophylactically in these patients, unless there is evidence of bleeding. Prophylactic platelet transfusion is indicated in preparation for invasive procedures in thrombocytopenic patients. Most invasive procedures (placement of central venous catheter, transbronchial biopsy, gastrointestinal endoscopy, and biopsy) can be performed with platelet counts greater than 40,000 to 50,000/μL. A platelet count of 20,000/μL is adequate for lumbar puncture and bone marrow biopsy, although BCSH recommended platelet counts higher than 50,000/μL for lumbar puncture and no platelet transfusion for bone marrow biopsy regardless of the severity of thrombocytopenia, providing adequate surface pressure is applied to the biopsy site. Circulating red blood also influences platelet function by scavenging endothelial cell nitric oxide, a vasodilating agent that inhibits platelet function. The venous blood hematocrit has been shown to correlate inversely with the bleeding time. Furthermore, anemia increases the shear stress and nitric oxide production by endothelial cells at sites of injury. Correcting the anemia in thrombocytopenic patients has been proposed to improve platelet function.
There are several studies on determining the optimal dose of prophylactic platelets. A randomized controlled trial comparing standard-dose (3–6 × 10 11 ) and low-dose (1.5–3 × 10 11 ) platelet transfusion to patients that expected to have platelet counts less than 10,000/mL for 10 days (strategies for transfusion of platelets [SToP] trial) did not support any benefit from low-dose strategy, which required more frequent transfusion episodes and was associated with a higher rate of severe bleeding, although the higher rate of bleeding did not reach statistical significance because of an early termination of the trial due to safety concerns. In another prospective randomized study assessing the efficiency of transfusing 2 doses (0.5 × 10 11 /10 kg vs 1 × 10 11 /10 kg), it was found that the higher dose resulted in a longer transfusion interval and decreased the number of transfusion episodes. Although this practice may improve quality of life by decreasing the frequency of transfusions, there was no clinically significant impact on bleeding complications. Furthermore, administering more platelets may diminish endogenous thrombopoiesis as platelets bind to thrombopoietin. In the Platelet Dose Study (PLADO trial) low, medium, and high doses of platelets were compared (1.1 × 10 11 , 2.2 × 10 11 , and 4.4 × 10 11 per square meter of body surface, respectively). Low-dose strategy was associated a decreased number of platelets transfused but an increased number of transfusion episodes per patient. There was no difference regarding bleeding incidences between low-platelet and high-platelet dose groups, in contrast to the concern raised by the StoP trial. Another important result of the PLADO trial was that the characteristics of transfused platelets, such as source (apheresis or random donors), ABO compatibility, and duration of storage before transfusion, had an impact on the posttransfusion increment in platelet counts but no significant effect on the prevention of bleeding. A higher increment in platelet counts after transfusion of apheresis or ABO-matched platelets was shown by several other studies even before completion of the PLADO trial.
Despite the common practice of prophylactic platelet transfusion, it has not been proven that this approach is superior to the therapeutic-only strategy, which limits transfusion of platelets only to bleeding in thrombocytopenic patients. A retrospective study on 3000 thrombocytopenic patients did not find any relationship between the morning platelet count or the lowest platelet count and the risk of bleeding on that day. An ongoing prospective randomized study on thrombocytopenic patients with hematologic malignancies will compare the safety of therapeutic-only approach to prophylactic strategy (TOPPs trial). Bleeding patients with thrombocytopenia should receive platelet transfusion with the goal of achieving a platelet count greater than 50,000/μL, except in patients who underwent cardiopulmonary bypass. Platelet functions are abnormal in these patients and they should receive platelets even when their platelet counts are greater than 50,000/μL. In uremic patients with bleeding or an anticipated invasive procedure, correction of uremia by dialysis and administration of 1-deamino-8-D-arginine vasopressin or cryoprecipitate are recommended. Platelet transfusion can be considered in these patients if the other interventions are ineffective.
Complications of platelet transfusions
Transfusion of all blood products including platelets can cause viral infection, such as hepatitis B, C, West Nile virus, and HIV, although the modern nucleic acid–based testing has reduced the risk to a very low level. Bacterial infection has been discussed previously in this article. The noninfectious complications such as volume overload, febrile nonhemolytic transfusion reactions (FNHTR), transfusion-related acute lung injury, and alloimmunization are the most common after platelet transfusion.
Allergic reactions are often seen with platelet transfusion and manifest as itching and urticaria in the absence of fever. Allergic reactions are caused by soluble substances present in donor plasma and mediated by the Immunoglobulin E response and histamine release in recipients. Administration of antihistamine reagents such as 25 to 50 mg diphenhydramine before platelet transfusion can reduce the frequency of allergic reactions. Temporary discontinuation of platelet transfusion and administration of diphenhydramine is adequate in the treatment of mild allergic reactions. In the absence of fever or unstable vital signs, transfusion of the same platelet unit can be resumed. If the allergic reaction reoccurs, a new unit of platelet should be transfused. In the presence of recurrent allergic reactions to different units of platelet products and after an anaphylactic reaction, removal of plasma and washing of platelet should be considered. Severe anaphylaxis after transfusion is rare and can occur in recipients who are deficient in Immunoglobulin A, C4, or haptoglobin. Treatment of an anaphylactic reaction after platelet transfusion requires administration of epinephrine, corticosteroid, and vasopressors and airway support.
Another common complication associated with platelet transfusion is FNHTR. Since the introduction prestorage leukocyte reduction, the incidence of FNTHR has decreased. FNHTR can manifest as fever, chills, nausea, vomiting, and dyspnea during transfusion, but can also occur minutes or hours after completion of transfusion. Most FNHTR are due to cytokines released from contaminated neutrophils in stored platelet products that accumulate during storage. Fresh platelet units (less than 2 days old) and removal of plasma decrease the frequency of FNHTR. In a minority of cases (6%), the recipient’s antileukocyte antibodies reacting to donor neutrophils cause FNHTR. In the presence of fever, it is difficult to differentiate between FNHTR, acute hemolytic reaction, and sepsis. As a result, transfusion of platelets coinciding with fever should be discontinued and not restarted with the same unit. Administration of meperidine is very effective in reducing chills, but antihistamine reagents are ineffective in FNHTR and corticosteroid only acts after a few hours. In the absence of the resolution of fever, the possibility of bacterial infection should be investigated.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree