PLACENTAL COMPARTMENT
In mammals, especially humans, the placenta has evolved into a complex structure that delivers nutrients to the fetus, produces numerous steroid and protein hormones, and removes metabolites from the fetus to the maternal compartment. The structure of the placenta is discussed in Chapter 111
PROGESTERONE
The principal source of progesterone during pregnancy is the placenta, although the corpus luteum is the major source during the first 6 to 8 weeks of gestation,1 when progesterone is essential for the development of a secretory endometrium to receive and implant a blastocyst. Apparently, the developing trophoblast takes over as the principal source of progesterone by 8 weeks, since removal of the corpus luteum before this time, but not after, leads to abortion.2 After 8 weeks, the corpus luteum contributes only a fraction of the progesterone secreted.
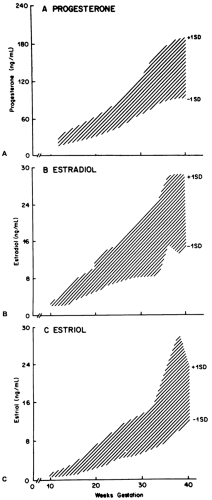
The placenta of a term pregnancy produces ˜250 mg progesterone each day. Maternal progesterone plasma levels rise from 25 ng/mL during the late luteal phase to 40 ng/mL near the end of the first trimester to 150 ng/mL at term (Fig. 108-1A). Most progesterone (90%) secreted by the placenta enters the maternal compartment.
The placenta of a term pregnancy produces ˜250 mg progesterone each day. Maternal progesterone plasma levels rise from 25 ng/mL during the late luteal phase to 40 ng/mL near the end of the first trimester to 150 ng/mL at term (Fig. 108-1A). Most progesterone (90%) secreted by the placenta enters the maternal compartment.
Although the placenta produces large amounts of progesterone, it normally has very limited capacity to synthesize precursor cholesterol from acetate. Radiolabeled acetate is only slowly incorporated into cholesterol in placental trophoblasts, and the activity of the rate-limiting enzyme of cholesterol biosynthesis—HMG-CoA reductase—in placental microsomes is low. Therefore, maternal cholesterol, in the form of low-density lipoprotein (LDL) cholesterol, is the principal substrate for the biosynthesis of progesterone.3,4 LDL cholesterol attaches to its receptor on the trophoblast and is taken up and degraded to free cholesterol, which then is converted to progesterone and secreted. These findings not only have provided new insights into the biochemical basis for placental progesterone formation, but they have also provided clues to other aspects of maternal-placental physiology. For example, the rate of progesterone secretion may depend on the number of LDL receptors on the trophoblast and may be independent of placental blood flow: (a) Cholesterol side-chain cleavage by the placental mitochondria is in a highly activated state, perhaps meaning that the placenta is under constant trophic stimulation and that hCG and GnRH produced by the placenta are the trophic substances; (b) cholesterol synthesis from acetate is limited, as discussed earlier; (c) the fetus does not contribute precursors for placental biosynthesis; and (d) the levels of maternal LDL are not rate-limiting for the placental products of cholesterol or progesterone.5
A functioning fetal circulation is unimportant for the regulation of progesterone levels in the maternal unit. In fact, fetal death, ligation of the umbilical cord, or anencephaly—which all are associated with a decrease in estrogen production—have no significant effect on progesterone levels in the maternal compartment.6,7
The physiologic role of the large quantity of progesterone includes binding to receptors in uterine smooth muscle, thereby inhibiting contractility and leading to myometrial quiescence. Progesterone also inhibits prostaglandin formation, which is critical in human parturition8 (see Chap. 109). Progesterone is essential for the maintenance of pregnancy in all mammals, possibly because of its ability to inhibit the T-lymphocyte cell-mediated responses involved in graft rejection. The high local levels of progesterone can block cellular immune response to foreign antigens such as a fetus, creating immunologic privilege for the pregnant uterus.9
ESTROGEN
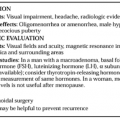
During human pregnancy, the rate of estrogen production and the levels of estrogen in plasma increase markedly (Fig. 108-1B and Fig. 108-1C), and the levels of urinary estriol increase 1000-fold.10 In fact, it has been estimated that during a pregnancy, a woman produces more estrogen than a normal ovulatory woman could produce in 150 years!5 The corpus luteum of pregnancy is the principal source of estrogen during the first few weeks; subsequently, nearly all of the estrogen is formed by the trophoblast of the placenta.
The mechanism by which estrogen is synthesized by the placenta is unique (Fig. 108-2). The placenta cannot convert progesterone to estrogens because of a deficiency of 17α-hydroxylase (CYP17). Thus, it must rely on androgens produced in the maternal and fetal adrenal glands. Estradiol-17β and estrone are synthesized by the placenta by conversion of dehydroepiandrosterone sulfate (DHEAS) that reaches it from both the maternal and the fetal blood. Near term, 40% of the estradiol-17β and estrone is formed from maternal DHEAS and 60% of the estradiol-17β and estrone arises from fetal DHEAS precursor.11 The placenta metabolizes DHEAS to estrogens through placental sulfatase, Δ4,5-isomerase and 3β-hydroxysteroid dehydrogenase, and aromatase enzyme complex. Estriol is synthesized by the placenta from 16α-hydroxydehydroepiandrosterone sulfate (16α-OHDS) formed in the fetal liver from circulating DHEAS. At least 90% of urinary estriol ultimately is derived from the fetal adrenal gland,11 which secretes steroid hormones at a high rate, sometimes up to 100 mg per day, mostly as DHEAS. The principal precursor for this DHEAS is LDL cholesterol circulating in fetal blood. A minor source is formation from pregnenolone secreted by the placenta. Only 20% of fetal cholesterol is derived from the maternal compartment, and because amniotic fluid cholesterol levels are negligible, the principal source of cholesterol appears to be the fetus itself. The fetal liver synthesizes cholesterol at a high rate and may supply sufficient cholesterol to the adrenals to maintain steroidogenesis.12
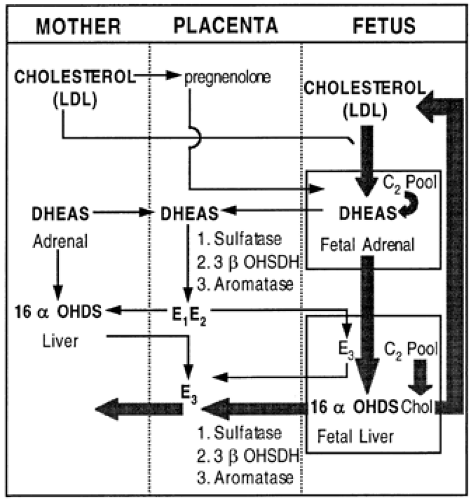 FIGURE 108-2. Sources of estrogen biosynthesis in the maternal-fetal-placental unit. (LDL, low-density lipoprotein; chol, cholesterol; OHDS, hydroxydehydroepiandrosterone sulfate; OHSDH, hydroxysteroid dehydrogenase; C2 pool, carbon-carbon unit; DHEAS, dehydroepiandrosterone sulfate; E1, estrone; E2, estradiol-17β7; E3, estriol.) (From Carr BR, Gant NE. The endocrinology of pregnancy-induced hypertension. Clin Perinatol 1983; 10:737.)
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|