PHYSIOLOGY OF THE OVARY
In women, the cyclic changes that occur in the menstrual cycle reflect structural and functional changes that occur within the follicle and corpus luteum. The dominant follicle begins as a primordial follicle and is slowly prepared for ovulation and luteinization by the action of the pituitary gonadotropins and ovarian growth factors. To understand the relationship of the dominant follicle to the events occurring in the menstrual cycle, the underlying physiologic mechanisms of folliculogenesis—recruitment, selection, atresia, ovulation, and luteogenesis (i.e., luteinization, luteolysis)—must be considered.
NORMAL FOLLICULOGENESIS
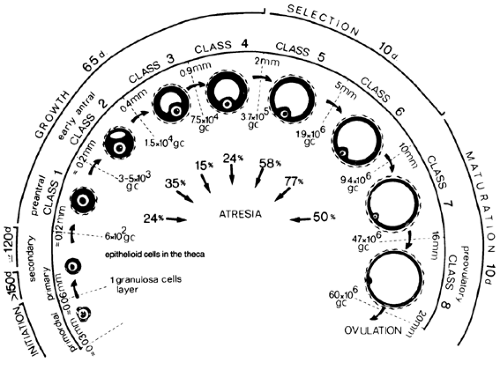
Follicular growth and development in women is a very long process.4 In each menstrual cycle, the ovulating follicle originates
from a primordial follicle that was recruited to grow ˜1 year earlier (Fig. 94-13). At first, the recruited primordial follicle develops very slowly, requiring ˜270 days to complete the preantral period and grow to the early tertiary stage (˜0.4 mm). The basis of this slow growth is the very long doubling time (˜250 hours) of the granulosa cells.38
from a primordial follicle that was recruited to grow ˜1 year earlier (Fig. 94-13). At first, the recruited primordial follicle develops very slowly, requiring ˜270 days to complete the preantral period and grow to the early tertiary stage (˜0.4 mm). The basis of this slow growth is the very long doubling time (˜250 hours) of the granulosa cells.38
When FSH enters the microenvironment of the early tertiary follicle, follicular fluid production by the granulosa cells is increased, and the graafian follicle begins to expand.18 During the antral period, a graafian follicle may pass through the small, medium, and large stages (see Fig. 94-13). The follicles that survive to ovulate require ˜85 days to complete the antral period (see Fig. 94-13).
Selection of the dominant follicle is one of the last steps in this long process. The dominant follicle is selected from a cohort of rapidly growing small graafian follicles in the late luteal phase of the menstrual cycle.4,38,39 It requires ˜15 to 19 days for the dominant follicle to complete its growth to the ovulatory stage (see Fig. 94-13). The 99.9% of all growing follicles that are not selected die by atresia (see Fig. 94-13).
RECRUITMENT
In women, recruitment is a continuous process throughout life, and the mean age for total follicular exhaustion is ˜51 years of age.3,40,40a The first indication that a primordial follicle has been recruited to grow is that the granulosa cells transform from a squamous to a cuboidal shape.41 As the granulosa cells round up, they acquire the ability for DNA synthesis and division, albeit at a very slow rate.41 When more than 90% of the granulosa cells are cuboidal, there occurs a dramatic increase in RNA synthesis in the arrested oocytes.25 The increased transcription and translation lead to the marked growth of the oocyte that occurs during preantral follicle development (see Fig. 94-3). The fact that changes in the oocyte begin later than those in the granulosa suggests that the granulosa cells might produce or respond to a ligand that initiates the recruitment of the primordial follicle into the growing pool of follicles.
What is known about the mechanisms of recruitment? The evidence that recruitment continues in the absence of pituitary gonadotropins argues that the process is regulated by intrinsic ovarian factors.3 Based on experiments in animals, it appears that the number of recruited primordial follicles varies with age—the highest level of recruitment occurs early in life, after which it decreases progressively with advancing age. This implies that the rate of recruitment is somehow determined by the actual number of primordial follicles and that the rate of recruitment can be suppressed by testosterone, thymectomy, starvation, and opioid peptides.3 Although inconclusive, these results suggest that recruitment is an active process that can be modulated (i.e., inhibited) by ligands. What evokes or triggers a particular follicle to grow is totally obscure.
It is noteworthy that a monotropic rise in FSH (perhaps due to reduced plasma inhibin levels) occurs in women during aging, and this increase in FSH coincides with an accelerated loss in
primordial follicles or ovary reserve (OR).3 In this regard, studies of aging rats indicate that the monotropic rise in plasma FSH may be involved in the accelerated loss of OR in old rats.3 This phenomenon could have clinical implications, because reduced OR leads to reduced fecundity in older women. Precisely how FSH might accelerate primordial follicle (OR) loss during aging is unknown, but it may involve the premature expression and/or activation of FSH receptors in granulosa cells of primordial follicles in aging ovaries.
primordial follicles or ovary reserve (OR).3 In this regard, studies of aging rats indicate that the monotropic rise in plasma FSH may be involved in the accelerated loss of OR in old rats.3 This phenomenon could have clinical implications, because reduced OR leads to reduced fecundity in older women. Precisely how FSH might accelerate primordial follicle (OR) loss during aging is unknown, but it may involve the premature expression and/or activation of FSH receptors in granulosa cells of primordial follicles in aging ovaries.
SELECTION
Morphometric studies of normal human ovaries indicate that the dominant follicle that will ovulate its egg the next cycle is selected from a cohort of small graafian follicles (4.7 ± 0.7 mm in diameter) at the end of the luteal phase of the menstrual cycle.4,38,39 After the midluteal phase, there is an approximate doubling of the rate of mitosis in the granulosa cells of all cohort follicles. This suggests that the demise of the corpus luteum is followed by a dramatic stimulation of mitosis and granulosa cell division in the cohort follicles.
The first visible sign that one of the follicles has been selected is that the granulosa cells of the chosen follicle maintain a high rate of mitosis, but the mitotic rate falls significantly in the other follicles of the cohort.4,38,39 This change becomes evident in the late luteal phase. The newly selected dominant follicle continues to grow and expand during the follicular phase and at a relatively rapid rate: 6.9 ± 0.5 mm at days 1 to 5; 13.7 ± 1.2 mm at days 6 to 10; and 18.8 ± 0.5 mm at days 11 to 14. The growth is caused by a progressive increase in follicular fluid and granulosa cell number.18 As the dominant follicle undergoes its growth and development, the cohort of nondominant follicles becomes increasingly more atretic, and rarely does an atretic follicle reach ≥9 mm in diameter over the cycle.39
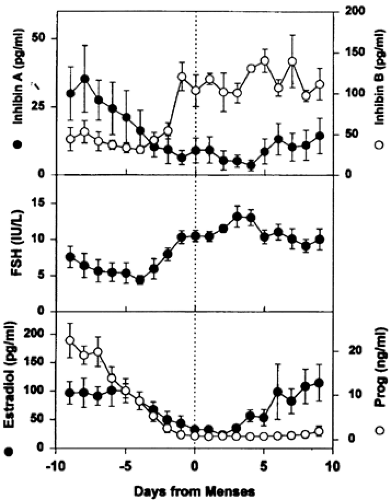
What do we know about the mechanisms underlying the selection process? There is compelling evidence that the secondary rise in FSH plays a central role in the selection process in rats.42 In women, the secondary rise in plasma FSH begins at or about the time plasma progesterone levels fall to basal levels at the end of the luteal phase, and it continues through the first week of the follicular phase43 (see Fig. 94-14). Evidence that suppression of the secondary rise in FSH prevents ovulation in monkeys supports the proposition that the secondary increase in FSH is critical for the continued growth and development of the dominant follicle in the primate.44 It seems likely that the secondary rise in FSH is also critical for selection in women, but further proof is needed.
During this period, the concentration of FSH increases steadily in the microenvironment of the chosen follicle, and FSH levels become low or absent in the nondominant follicles.45 In the dominant follicle, the FSH stimulates a sharp increase in the number of granulosa cells and apoptosis is suppressed. By contrast, the growth development of nondominant follicles is suppressed in the absence of adequate levels of FSH, and apoptosis is activated in the granulosa cells. In this way, the selected follicle achieves dominance. How is dominance established? In studies in the monkey, estradiol has been found to be a causative agent by virtue of negative feedback on the pituitary gonadotrope.42,44 In this concept, the high intrafollicular levels of FSH lead to increased estradiol production, which, in turn, suppresses FSH secretion by the pituitary. When FSH becomes rate-limiting in the nondominant cohort follicles, they undergo atresia.38 Human menopausal gonadotropins can stimulate granulosa cells to divide mitotically in nondominant follicles during the early follicular phase.38 If FSH levels within the microenvironment are increased, it appears that the nondominant follicle can be rescued from atresia.38 This rescue phenomenon could be involved in the generation of multiple large follicles in women undergoing ovulation induction with exogenous FSH.
The FSH rise that begins before the onset of menses also occurs with concomitant decreases in inhibin A produced by the corpus luteum.43 The inverse relationship between inhibin A and FSH suggests an endocrine role for inhibin A in the regulation of the secondary FSH rise (see Fig. 94-14). In contrast to inhibin A, inhibin B increases before menses, coincident with the FSH rise (see Fig. 94-14). Although estradiol secretion by the follicle is primarily responsible for the negative-feedback regulation of FSH during the follicular phase of the cycle, it is possible that inhibin B produced by graafian follicles might play a role as well. However, the role of inhibin B in reproduction remains unknown. It should be mentioned that there is considerable interest in follistatin because it can specifically bind to activin and inhibin and modulate their activity.46 Follistatin might, therefore, be a physiologically relevant protein in the control of folliculogenesis in the ovary. However, further work is needed to establish this concept.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree