Fig. 1
Adrenal anatomy and blood supply
Blood Supply
Arteries
The adrenal gland has three main groups of arteries carrying oxygenated blood to the cortex and medulla [10]. The superior adrenal arteries also known as superior suprarenal arteries (which originate from the inferior phrenic arteries), the middle adrenal arteries also known as middle suprarenal arteries (which originate from the aorta), and the inferior adrenal arteries also known as inferior suprarenal arteries (which originate from the right and left renal arteries) [10]. The arteries branch to form a plexus within the adrenal capsule to supply the cortex [10]. There are numerous minor branches that supply the gland such as branches from the intercostal arteries, the left ovarian artery and the left internal spermatic artery [10]. Some arterial vessels penetrate the medulla, which obtains additional blood supply from branches of arteries that supply the central vein and cortical tissue around this vein [10] .
Veins
Each adrenal gland is drained by a major adrenal vein [10]. The left adrenal vein (2–3 cm) is longer than the right adrenal vein [4].
Left adrenal vein: drains into the left renal vein typically after joining the left inferior phrenic vein [4].
Right adrenal vein: drains directly into the inferior vena cava as it exits the adrenal gland [4].
Lymphatic Drainage
There are two lymphatic plexuses, one in the cortex and one in the medulla [4]. Most lymphatic drainage from the adrenal glands goes to the lateral aortic lymph nodes and the para-aortic nodes and a minority of the lymphatic drainage terminates in the thoracic duct [4] . These lymphatic drainage patterns help explain the spread of malignant adrenal tumors [4] which commonly invade the inferior vena cava, para-aortic lymph nodes, liver, and lungs [9].
Nervous Innervation
Visceral afferent innervation from the celiac plexus, aorticorenal ganglia, and renal autonomic ganglia as well as the posterior vagus nerve, phrenic nerve, and greater and lesser splanchnic nerves are the main nervous system components that supply the adrenal glands [10]. These nerve fibers travel through the adrenal cortex and terminate in the medulla, where they function as postsynaptic sympathetic nerves, essentially enabling the adrenal medulla to function under neuroendocrine control in the secretion of catecholamines [10].
Microscopic Anatomy
Adrenal Cortex
The zona glomerulosa is approximately 15 % of the adrenal cortex and is responsible for aldosterone production [4]. It is composed of small, lipid-poor cells beneath the adrenal capsule. The zona fasciculata makes up 75 % of the cortex and is responsible for cortisol production [4] . It is composed of more lipid-rich cells than the zona glomerulosa, known as clear cells [4]. The zona reticularis makes up 10 % of the cortex, and is primarily responsible for androgen production, and has cells containing lipofuscin granules [4]. Both the zona fasciculata and zona reticularis are under the control of adrenocorticotropic hormone (ACTH) [4].
Adrenal Medulla
The adrenal medulla is composed of chromaffin cells [4]. These are large columnar cells with neurosecretory granules composed of catecholamines (epinephrine and norepinephrine) as well as proteins, lipids, chromogranins, neuropeptides, and proopiomelanocortin [4] . These cells are arranged in cords around a network of venous sinusoids that drain blood from the cortex [4].
Physiology
As the adrenal cortex develops from the mesoderm lining the abdominal wall and the adrenal medulla develops from neural crest cells, they develop distinct endocrine functions despite being one gland [1]. The unique functional aspects of each component layer of the adrenal gland will be explored in this section.
Adrenal Cortex
The adrenal cortex secretes three major hormones: aldosterone, cortisol , and adrenal androgens [4]. These are produced from cholesterol through complex biochemical pathways with numerous intermediate steps as outlined in Fig. 2. Low-density lipoprotein is the major source for the cholesterol supplied to the adrenal gland [4].
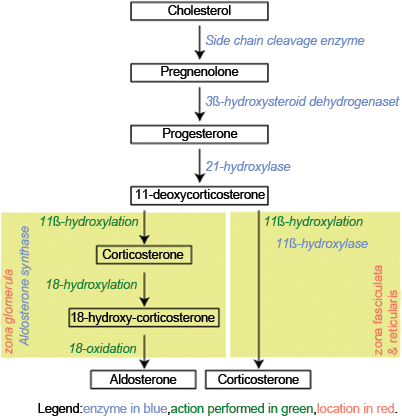

Fig. 2
Biosynthesis of aldosterone and cortisol. (Author: Jean-Iteinne Poirrier. Date: February 9, 2007. License: CC BY-SA 2.5, Creative Commons)
The zona glomerulosa produces aldosterone, which is under the regulation of the renin–angiotensin system [4]. The zona fasciculata and zona reticularis produce cortisol and adrenal androgens, respectively, under the regulation of ACTH [4]. Because the zona glomerulosa lacks the enzyme, 17α-hydroxylase, it cannot produce 17-hydroxypregnenolone, the precursor for adrenal androgens or 17-hydroxyprogesterone, the precursor for cortisol [5]. The zona fasciculata and reticularis have 17α-hydroxylase activity; therefore cortisol and adrenal androgens are produced exclusively by the zona fasciculata and reticularis [5]. The zona glomerulosa , by contrast, is the only part of the adrenal cortex in humans that has 18-hydroxycorticosterone activity, a key step in aldosterone production [12].
Zona Glomerulosa
Synthesis, Circulation, Action, Inhibition
Aldosterone, the predominant mineralocorticoid is produced by the zona glomerulosa and involves a close feedback loop with the renin–angiotensin system of the kidneys (Fig. 3) [13]. Stimuli for aldosterone secretion include elevated serum potassium levels, sympathetic stimulation, decreased pressure sensed by the renal afferent arteriole, a part of the tubuloglomerular feedback mechanism, and ACTH [14]. Hypotension or decreased blood flow is sensed by the macula densa, specialized cells in the distal convoluted tubule, which stimulates the juxtaglomerular apparatus to release renin, which causes angiotensinogen to be converted to angiotensin I [1]. Renin is produced by the juxtaglomerular cells of the kidney, which are microscopic structures located between the vascular pole of the renal corpuscle and the distal convoluted tubule of the same nephron, surrounding the glomerular afferent arterioles [15] . Next, angiotensin I is converted to angiotensin II by the enzyme, angiotensin-converting enzyme (ACE), an enzyme primarily found in the pulmonary vasculature [1]. Angiotensin II stimulates the adrenal glands to produce aldosterone, triggers the release of epinephrine and norepinephrine from the adrenal medulla , promotes the release of vasopressin or antidiuretic hormone, which increases water absorption by the kidney collecting duct and also functions as an arterial smooth muscle constrictor [1].
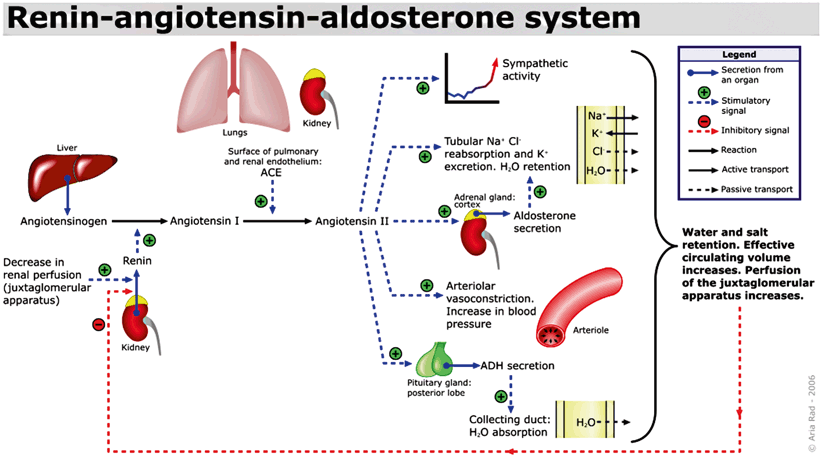

Fig. 3
Renin–angiotensin–aldosterone system. (Author: A. Rad. Date: February 2, 2006. License: GFDL-self Created using XaraX¹, Creative Commons)
Once aldosterone is released into the circulation, it has several effects that result in enhanced absorption of sodium and water and secretion of potassium and hydrogen ions [12]. Aldosterone binds to the mineralocorticoid receptor within cells of the renal cortical collecting ducts [16]. Aldosterone acts within 1 h of secretion to increase sodium channel activity on the apical membranes of cells in the cortical collecting tubule, increasing apical membrane permeability for sodium [12]. Via a sodium potassium adenosine triphosphatase (ATPase) pump, aldosterone acts on the principal cells to promote sodium absorption and potassium excretion. At the intercalated cell of the kidney’s cortical collecting tubule, aldosterone promotes the secretion of protons via an ATPase pump [16]. These effects augment the difference in electrical potential across the renal tubule, making the lumen more negative and thus more favorable for aldosterone-induced secretion of potassium and hydrogen ions, which carry a positive charge [4] .
Aldosterone secretion is inhibited by dopamine directly at the level of the adrenal gland [9]. Atrial natriuretic factor also directly inhibits dopamine and also blocks the stimulatory effects of angiotensin II, potassium, and ACTH [9]. Somatostatin inhibits aldosterone secretion by inhibiting angiotensin II [9].
Metabolism and Excretion
Hepatic metabolism reduces and conjugates aldosterone, which is then excreted in the urine [9]. People with impaired hepatic function from cirrhosis or congestive heart failure can have elevated aldosterone levels from decreased clearance [9].
Zona Fasciculata/Zona Reticularis
The zona fasciculata is the site of cortisol and the zona reticularis is the site of adrenal androgen production [1]. Cortisol is the major glucocorticoid but other glucocorticoids such as cortisone are produced by the adrenal cortex . Aldosterone, while a mineralocorticoid, does also exert some glucocorticoid effects [9].
Cortisol
Synthesis, Circulation, Action, Inhibition
The process leading to cortisol secretion by the adrenal cortex starts at the level of the hypothalamus in the midbrain. Corticotropin-releasing hormone (CRH), produced in the hypothalamus is secreted to the anterior pituitary gland where it stimulates the formation and secretion of ACTH from the precursor proopiomelanocortin [17].
ACTH secretion is pulsatile and is governed by circadian rhythms whereby ACTH secretion is high during the morning hours and becomes lower in the late evening through the early part of sleep, reaching its trough around 3–4 a.m. ACTH levels start to climb as people awaken in the early morning leading to a corresponding increase in cortisol levels in the early daytime [18]. This pattern can be disrupted by shift work where people change their sleep/wake cycles, by changes in physical stress such as starvation, exercise, critical illness, psychiatric illnesses, and illnesses that affect cortisol production, such as Cushing’s syndrome, or cortisol binding, such as liver disease [4]. ACTH binds to receptors throughout the zona fasciculata and zona reticularis through a G protein mechanism, whereby ACTH binding triggers a cascade of events involving an increase in cyclic adenosine monophosphate (cAMP) and activation of protein kinase A, which leads to more cortisol production from cholesterol [4]. ACTH controls the rate-limiting step of cortisol synthesis, the conversion of cholesterol to pregnenolone [1]. This allows feedback regulation where low cortisol levels trigger increased production of cortisol from cholesterol by upregulation of ACTH [4].
Most cortisol (~ 80 %) circulates bound to corticosteroid-binding globulin (CBG), which is produced by the liver [19]. About 10–15 % is bound to albumin and 10 % of circulating cortisol is free but this percentage can increase rapidly with stress [4, 20]. CBG also binds to progesterone, aldosterone, and 11-deoxycorticosterone [20]. CBG levels can increase, especially when estrogen levels are high such as during pregnancy or with oral contraceptive use [4]. Diabetes and hyperthyroidism will also increase CBG and thus increase circulating cortisol levels [4]. CBG levels decline with chronic steroid use and with various disease states including cirrhosis [20]. CBG binds to cortisol in a 1:1 ratio and controls the delivery of cortisol to organs and tissues [20].
Cortisol Function
Cortisol affects all major organ systems and biological processes. Free cortisol is the biologically active form [21]. The glucocorticoid receptor is present in nearly all cells and is a member of the steroid hormone receptor family of proteins [21]. Like all steroid receptors, once cortisol binds to the glucocorticoid receptor, it translocates to the cell nucleus and initiates transcription [21]. The steroid–receptor complex must dimerize to bind the glucocorticoid response elements, specific DNA sequences that control regulatory processes [9]. The glucocorticoid-responsive genes are regulated based on a complex interplay between the cortisol–glucocorticoid receptor complex and other transcription factors as well as through membrane-associated receptors and second messengers [21]. While glucocorticoids increase transcription of many genes, they suppress transcription of others [9].
At the level of the embryo, surfactant production is stimulated by glucocorticoids [9]. Glucocorticoids promote differentiation of neural crest cells into chromaffin cells in the developing adrenal medulla [9].
In the central nervous system, many cells are directly influenced by glucocorticoid binding. Mood, behavior, sleep patterns, and reception of sensory input are all modulated by glucocorticoids [22].
In the immune system, glucocorticoids block many inflammatory pathways including the number of T and B lymphocytes in the peripheral circulation [9], the phagocytic and cytotoxic functions of macrophages [9], and synthesis of arachidonic acid, a mediator of the vasodilatory inflammatory response that leads to the production of prostaglandins and thromboxanes [21]. Glucocorticoids inhibit fibroblasts and cause loss of connective tissue, thin skin, easy bruising, and delayed wound healing [1].
In the kidney, glucocorticoids enhance renal sodium retention, which leads to increased water absorption [21]. Additionally, glucocorticoids have weak mineralocorticoid activity and induce the angiotensin II receptor which results in increased aldosterone levels and sodium and water retention [21]. By contrast, atrial natriuretic factor also contains glucocorticoid-responsive elements and atrial natriuretic factor will increase in the presence of glucocorticoids [9]. This leads to greater excretion of salt and water [9].
In bone, glucocorticoids inhibit osteoblast function, which decreases new bone formation [9]. In children, glucocorticoids reduce chondrocyte proliferation and induce apoptosis, which is vital for longitudinal growth as chondrocytes take up calcium, secrete calcium phosphate and hydroxyapatite, and form mineralized bone [23]. In adults, glucocorticoids inhibit osteocalcin, an extracellular matrix protein that causes bone mineralization [23]. Glucocorticoids exert indirect effects on bone by decreasing intestinal calcium absorption and decreasing renal calcium reabsorption [24].
In the metabolism of lipids and glucose, glucocorticoids play a crucial role. They are responsible for both glycogen synthesis and hepatic gluconeogenesis [22]. They inhibit glucose transport into cells [25] to inhibit glucose utilization by peripheral tissues and promote lipolysis in adipose tissue [22, 26]. Food intake increases the glucocorticoid response and glucocorticoids may also regulate leptin, a hormone produced by adipose cells that regulates food intake at the central nervous system level [27]. Glucocorticoids promote leptin secretion by adipocytes and this may be one mechanism for increased body fat when people take oral glucocorticoids [28]. Glucocorticoids increase protein breakdown and urinary nitrogen excretion [1].
Glucocorticoids inhibit their own production by feedback inhibition at the pituitary level to block further ACTH secretion and they act at the hypothalamus to block CRH secretion [29]. If cortisol levels remain elevated due to exogenous glucocorticoid use, ACTH levels will decrease to a point where they are no longer responsive to stimulation [29]. This leads to suppression of CRH and ACTH release and atrophy of the adrenal cortex, which can be apparent on adrenal imaging such as computed tomography (CT) scan or magnetic resonance imaging (MRI) [29]. In addition to physiologic suppression, exogenous steroid use will also suppress the hypothalamic–pituitary–adrenal (HPA) axis and stop CRH and thus, ACTH secretion [29].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree