Phyllodes Tumor | 3 |
Jolinta Lin, Elizabeth Nichols, Susan B. Kesmodel, Dina Ioffe, Olga Ioffe, and Katherine H. R. Tkaczuk
EPIDEMIOLOGY
Phyllodes tumors (PTs) are uncommon, biphasic, fibroepithelial neoplasms of the breast that were originally named “cystosarcoma phyllodes” for their leaf-like papillary projections seen on histology. PTs account for approximately 0.5% of primary breast neoplasms (1,2). Population studies have estimated an incidence of 2.1 per 1 million women. Most PTs are benign, but some have malignant potential (18%–25%) (3–5). Thus, the broader term “phyllodes tumor” is used as opposed to the previous term of “cystosarcoma phyllodes.”
CLINICAL PRESENTATION
Although they can occur at any age, the majority of PTs present in women in their fourth decade of life and are most commonly detected as rapidly enlarging, painless, palpable breast masses. They tend to be larger and present 10 to 20 years later than the peak age for fibroadenomas. On imaging, these appear as round, sharply defined masses with cysts or clefts, and occasionally coarse calcifications (6,7).
PATHOLOGY
Like fibroadenomas, PTs arise from intralobular stroma, which distorts lobules and ducts and incorporates them within the mass; while the tumor contains epithelial elements, only the stromal component is neoplastic (3). Compared to fibroadenomas, PTs have increased cellularity of the stroma, a higher mitotic rate, and a leaf-like architecture with periepithelial stromal condensation, also known as cuffing. Histologically, PTs are characterized by elongated, branching epithelial cleft-like spaces within leaf-like hypercellular epithelial-lined stromal fronds that protrude into cystically dilated spaces, creating a staghorn appearance. Increased stromal cellularity is a characteristic feature of PT; this feature helps differentiate PTs from fibroadenomas and helps to histologically grade PTs.
PATHOLOGIC CLASSIFICATION OF PHYLLODES TUMORS
While many classification systems have been proposed for these neoplasms, none is universally honored. In 1951, Treves and Sunderland classified PTs as benign, borderline, or malignant based on histological parameters (8), a classification system that is still most widely used to date. In 2003, the World Health Organization (WHO) proposed a classification system based on Treves and Sutherland’s three categories, only with better defined criteria, which were amended in 2012 (9). The histological parameters in this classification system include the degree of stromal cellularity and atypia, mitotic count, stromal overgrowth (defined as presence of stroma without epithelium in at least one low-power field), and invasion into the surrounding breast tissue (10).
BENIGN PHYLLODES TUMORS
Benign PTs comprise 60% to 75% of all PTs and are characterized by mildly increased stromal cellularity, minimal stromal atypia, well-defined pushing borders, <5 mitoses/10 high power field (HPF), and no stromal overgrowth. Although they are not likely to metastasize, they have the potential for local recurrence (LR), making it important to distinguish them from fibroadenomas. Many of these histologic features overlap with fibroadenomas (pushing/circumscribed borders and modest stromal cellularity), making it particularly difficult to distinguish the two on core needle biopsy, largely due to limited sample size. The key features distinguishing PTs from fibroadenomas are the characteristic leaf-like structures and increased stromal cellularity around the epithelial clefts—stromal cuffing (Figure 3.1).
MALIGNANT PHYLLODES TUMOR
On the other end of the spectrum are malignant PTs, which account for 10% to 20% of all PTs. They have marked cellular atypia (coarse chromatin, significant variation in nuclear size, and irregular membranes with discernible nucleoli), moderate to marked stromal atypia, >10 mitoses/10 HPF, infiltrative borders, and stromal overgrowth. All of these features must be present to be graded as a malignant PT; otherwise, it is considered a borderline PT (Figure 3.2). Alternatively, a PT is graded as malignant if a heterologous element is present (eg, liposarcoma, chondrosarcoma, osteosarcoma), regardless of the other parameters.
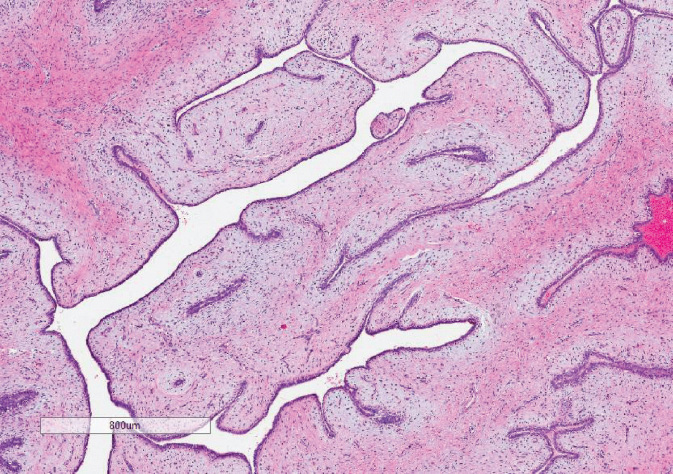
Figure 3.1 Benign phyllodes tumor. Note the leaf-like architecture and stromal condensation under the epithelial lining of the spaces.
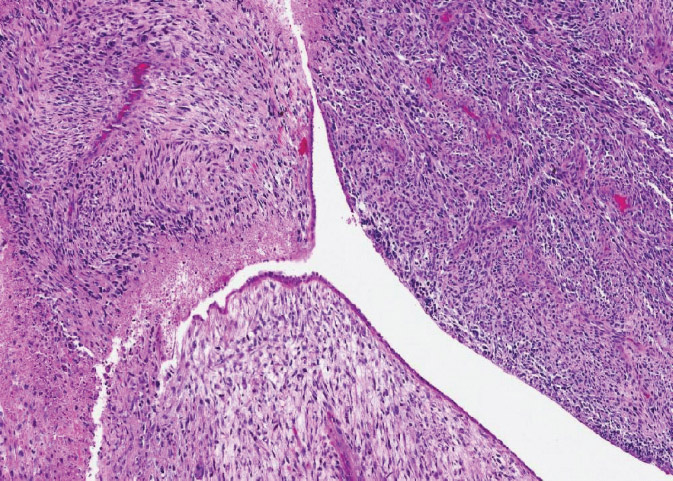
Figure 3.2 Malignant phyllodes tumor. The leaf-like architecture is still present but stroma is very hypercellular, highly pleomorphic, with necrosis and frequent mitotic figures.
BORDERLINE PHYLLODES TUMOR
As their name implies, borderline PTs fall in between these two extremes, making precise definitions difficult. They comprise 15% to 20% of all PTs and generally have intermediate features or do not fully meet malignant criteria. They have moderate stromal cellularity, mild to moderate stromal atypia, 5 to 9 mitoses/10 HPF, and may have focal infiltrative borders. Predicting tumor behavior based on these histologic parameters alone has proved futile, as benign PTs have been known to metastasize while malignant ones may neither recur nor metastasize after wide local excision. However, stromal overgrowth is consistently associated with aggressive behavior and metastatic potential. There have been efforts to identify tumor markers as predictors of clinical course and outcome, including Ki-67, p53, CD117, and CD34, but all have failed to consistently and reliably predict outcomes. The major clinical concern is LR, which can be seen with all types of PTs, and is best mitigated with adequate wide local excision (3,6,11,12).
DIAGNOSIS
Core needle biopsy is the preferred method of evaluating breast lesions; however, the limited specimen from a core biopsy can make it difficult to accurately distinguish a benign PT from a fibroadenoma (3,13).
TREATMENT
Wide local excision of the mass with a margin of normal breast tissue remains the standard of care for PT (6); total mastectomy is generally reserved for large tumors where breast conserving surgery (BCS) would not lead to an acceptable cosmetic outcome or for cases of LR when a reexcision is not possible (5,14). Routine axillary staging or lymph node dissection is not recommended for PT since the incidence of axillary lymph node metastasis is low (6,14). Negative margins, wide excision (microscopic margins ≥1 cm), and mastectomy can have high rates of local control (80%–100%) (6,14,15). Some groups report better local control with total mastectomy for patients with borderline or malignant PT (5,16).
PROGNOSIS
Given the rarity of PT in general, most studies that have examined local control and survival rates have been either retrospective single-institution studies or population-based studies. Population-based studies have noted high rates of cancer-specific survival even for malignant PTs, with 15-year cancer-specific survival of 89% (1). Other groups have combined borderline and malignant PTs together and estimated 10-year disease-free survival (DFS) and overall survival (OS) rates to be 68% and 88%, respectively (5). Histopathologic factors associated with disease recurrence include the degree of stromal hypercellularity (17), stromal atypia, and permeative margins (11), although this is not consistent in all reported studies (6). While few patients develop metastatic disease, some groups have noted that patients with infiltrating tumor margin, severe stromal overgrowth (14), atypia, and cellularity are at higher risk for metastasis (6). Belkacémi et al collected data from 443 women in the Rare Cancer Network with PT and reported LR of 19% and incidence of distant metastases of 3.4% at a median follow-up of 106 months (5). Patients with borderline or malignant PT were more likely to develop LR than patients with benign PT, with 10-year local control rates of 64% and 87%, respectively (P < .0001). Adjuvant radiation therapy (RT) in patients with borderline or malignant PT also significantly increased local control rates compared to BCS alone.
Benign PT carries a good prognosis even when treated with surgery alone; most LRs are salvaged by secondary surgery, likely contributing to the excellent survival rates. Belkacémi et al observed LR rates of 13% at 10 years in women with benign PT (5). Kim et al reported even fewer LR (3.4%) among 143 women with benign PT regardless of surgical margins or adjuvant radiation (RT) (16). Our approach for the management of benign PT is wide local excision to obtain negative pathologic margins with no adjuvant therapy.
RADIATION THERAPY FOR BORDERLINE AND MALIGNANT PHYLLODES TUMOR
Patients with borderline and malignant tumors have higher rates of LR after surgery than benign PT, 18% to 26% and 36% to 47%, respectively (5,16). In women with borderline and malignant PT who opt for BCS, improvement in local control rates may be observed with adjuvant RT (5,18). Given the rarity of borderline and malignant PT, the role of adjuvant RT has not been well established. Prior to 2009, most studies reported surgery as the primary treatment, with approximately 9% of PTs treated with adjuvant RT (1,5,19). A Surveillance, Epidemiology, and End Results (SEER) analysis of patients with malignant PT suggested that patients who received adjuvant RT had worse cause-specific survival compared to those who had surgery alone; however, the authors note that only a small percentage of patients (9%) received adjuvant RT, which would suggest selection bias that the worst tumors likely received adjuvant RT (1). The authors recognize that important clinical and pathologic data such as presence of stromal overgrowth, tumor necrosis, tumor grade, margin status, lymph node status, histologic subtype, hormone receptor status, specifics of the surgical and radiotherapy procedures, and clinical reasoning for mastectomy versus wide excision were not available or were incomplete within the SEER record (1).
A recent meta-analysis focusing on borderline and malignant PT showed a lower relative risk of LR with adjuvant RT with an absolute risk difference of 10.1% (20). Despite a clear reduction in LR in the patients who had BCS, no statistically significant differences were seen in OS or DFS between patients who received adjuvant RT and those who had surgery alone (20).
A population study using data collected from the National Cancer Data Base from 1998 to 2009 revealed that the use of adjuvant RT for PT has been increasing over recent years. Use of RT doubled over the study period from 9.5% (1998–1999) to 19.5% (2008–2009). Among the 3,120 women with malignant PT, women were significantly more likely to receive RT if they were diagnosed later in the study, were age 50 to 59 years old, had tumors >10 cm, or had lymph nodes removed. While recurrence data was only available for 1,774 patients, the overall recurrence rate was 14.1%, and LR was 5.9%. The multivariate model demonstrated that adjuvant RT significantly reduced LR (hazard ratio 0.43, 95% CIs [0.19, –0.95]) (19).
Pezner et al from the City of Hope published local control rates for 478 patients with malignant PT undergoing surgery alone with the important finding that tumor size was associated with tumor recurrence. With a median follow-up of just over 5 years, the recurrence rate following a lumpectomy was 9% for tumors <2 cm, 15% for tumors between 2 and 5 cm, and 41% for tumors >5 cm. Based on this data, we recommend whole breast radiation to 50 Gy at 1.8 to 2 Gy per fraction followed by a boost to the tumor cavity for an additional 10 Gy for tumors >5 cm following a BCS with negative margins (15).
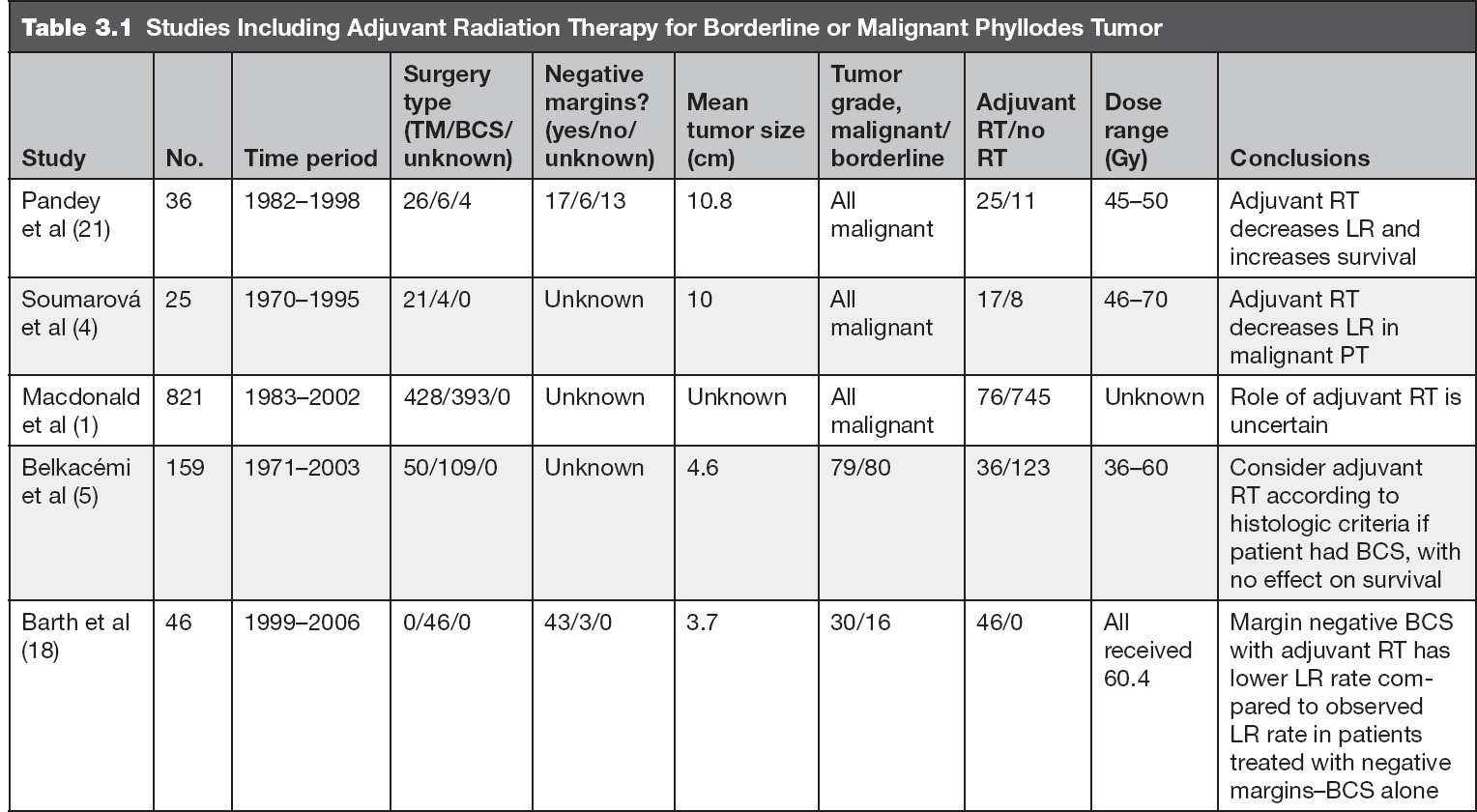
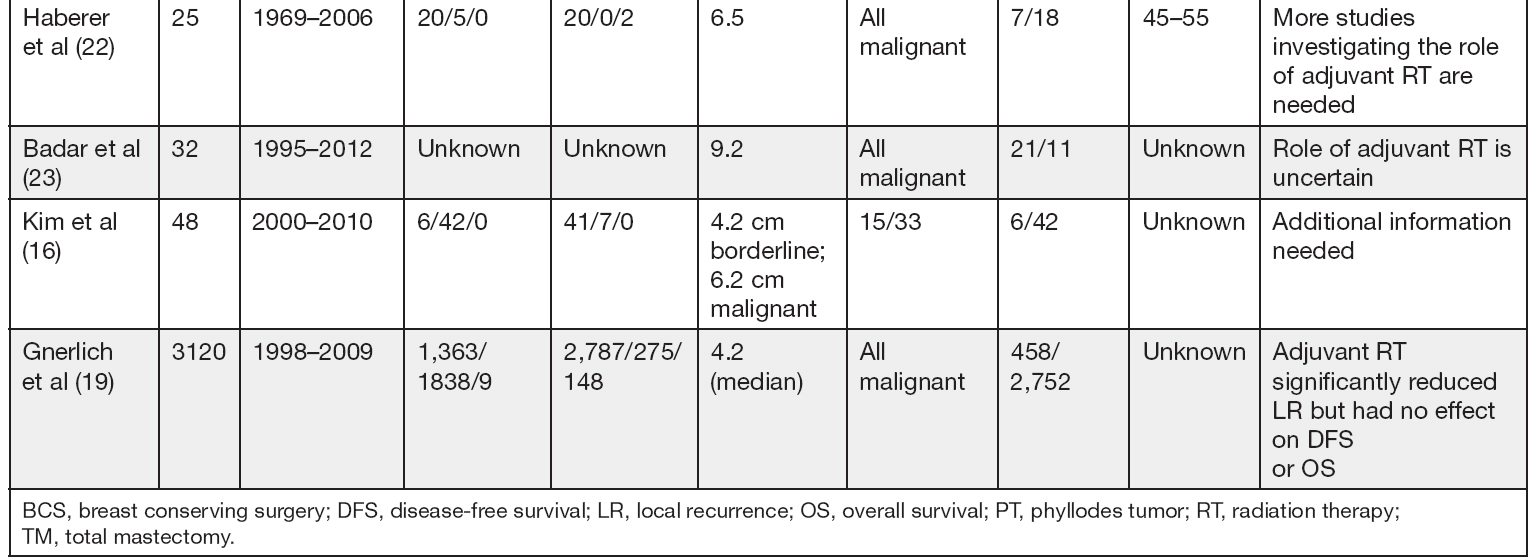
Adjuvant RT to the chest wall after mastectomy may also be performed in patients with malignant PT, especially when the tumor is large; however, the benefit is not completely clear given the limited data (4,15). In Table 3.1 we summarize studies including adjuvant RT for borderline or malignant PT.
CHEMOTHERAPY
A role for adjuvant chemotherapy in patients with borderline or malignant PT has not been established and remains controversial. Since most retrospective analyses report excellent OS and low rates of systemic recurrence, it is unlikely that the use of systemic chemotherapy for management of adequately resected PT is beneficial. Due to the low incidence of this malignancy, it is also unlikely that prospective clinical trials will be conducted to address the role of adjuvant chemotherapy in management of PT. Genetic tumor analysis will supplement classical histologic examination and may potentially identify targetable mutations in order to improve our management of these rare tumors.
A small prospective observational study in 28 patients with malignant PT reported outcomes in patients who were assigned to chemotherapy or observation. Seventeen patients received adjuvant chemotherapy consisting of four cycles of 65 mg/m2 doxorubicin infusion over 48 hours and 960 mg/m2 dacarbazine infusion over 48 hours. Eleven patients were in the observation group. All patients had surgical resection, 38% had an axillary lymph node dissection, and 25% received adjuvant RT. The median age was 42 years (range, 23–76 years) and median tumor size was 13 cm (range, 3–30 cm). With median follow-up of 15 months (range, 2–81 months), 7 recurrences and 5 deaths were observed. The 5-year recurrence-free survival rate was 58% (95% CI = 36% and 92%) for the patients who received adjuvant therapy and 86% (95% CI = 63% and 100%) for the patients who did not (P = .17). The median survival after recurrence was 6.5 months. The authors concluded that adjuvant chemotherapy with doxorubicin and dacarbazine did not affect recurrence-free and OS, although there was a clear selection bias for which patients received chemotherapy in this study. In addition, the choice of dacarbazine instead of ifosfamide is considered less standard since ifosfamide plus doxorubicin is superior to dacarbazine plus doxorubicin in treatment of other soft tissue sarcomas (24).
Another unresolved management issue is whether systemic chemotherapy may have clinical benefit in those patients who develop LR after initial adequate surgical resection or local or systemic recurrence after surgical resection followed by local RT. Again we found no conclusive evidence that systemic chemotherapy is of benefit and management should be considered on a case-by-case basis as the majority of available information is from case reports of clinical response after systemic chemotherapy for recurrent or metastatic disease; agents used in this setting include cisplatin, etoposide, or ifosfamide (25–28).



