Peripheral T Lymphocyte Responses and Function
Marc K. Jenkins
INTRODUCTION
This chapter focuses on the processes by which conventional thymus-derived (T)-lymphocytes that express αβ T-cell antigen receptors (TCRs) become activated by their cognate peptide (p): major histocompatibility complex (MHC) ligand. It will cover how TCR ligation by foreign antigen-derived p:MHC ligands causes rare naïve T cells from the preimmune repertoire to proliferate in the secondary lymphoid organs (sometimes referred to by immunologists as the periphery) to become effector cells capable of producing microbicidal cytokines and eliminating foreign antigens from nonlymphoid tissues. It will also describe how the innate immune response and signals from costimulatory and cytokine receptors shape the functional and migratory properties of effector T cells, and ends with a discussion of how effector cells give rise to diverse memory cell subsets. An emphasis is placed on the dynamics and anatomy of naïve to effector to memory-cell conversion.
The chapter will focus on conventional T cells that express αβ TCRs. The reader is directed to other chapters in the book for details concerning natural killer T cells, γδ T cells, and regulatory T cells.
NAÏVE T CELLS
To understand effector T-cell generation, it is important to consider the naïve T cells from which they are derived. The capacity of the host to make effector and memory cells in response to new foreign proteins depends on the existence of naïve T cells that express αβ TCRs specific for MHC-bound peptides derived from that protein.1 Naïve T cells with this particular p:MHC specificity are rare members of a vast repertoire also containing naïve T cells specific for other p:MHC ligands. Each naïve T cell expresses a randomly generated TCR, which is produced regardless of whether the relevant foreign antigen is in the host’s body. Thus, naïve T cells are defined as T cells that have not yet been stimulated by a p:MHC ligand for which their TCR has a high affinity.
Generation
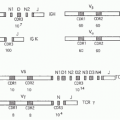
A naïve T cell is the end result of a complex developmental process that occurs in the thymus.2 To complete development, each thymocyte must produce an αβ TCR by randomly recombining one of multiple variable (V), diversity (D), and joining (J) deoxyribonucleic acid segments at the Tcrb locus and one of multiple V and J segments at the Tcra locus.3 Once an in-frame rearrangement at a locus has occurred on one chromosome, further rearrangement on the other chromosome is suppressed, although not completely. These processes lead to the situation where most developing thymocytes express a single unique type of αβ TCR.
Thymocytes that successfully express a TCR must then pass positive and negative selection based on the p:MHC specificity of that TCR.4 Positive selection occurs just after completion of TCR gene rearrangement at the time when thymocytes first express TCRs and coexpress both cluster of differentiation (CD)4 and CD8 coreceptors. To pass positive selection, a thymocyte must receive a TCR signal transduced by low affinity binding to a self p:MHC ligand on radio-resistant thymic epithelial cells in the cortex. It was thought that this process was inefficient due to the improbability of VDJ segment rearrangement by chance producing TCRs with any affinity for a self p:MHC ligand.5 However, recent work from Marrack and colleagues has shown that all TCRs have germ-line encoded complementarity determining region (CDR) 1 and CDR2 domains within their V segments that bind MHC molecules.6 This binding has been postulated to cause most thymocytes to undergo negative selection,7 which is a desirable outcome for the host because these cells could cause autoimmunity if allowed to mature. In this model, the only thymocytes that receive the weak positive selection signal are those that produce a CDR3 through VDJ recombination that partially impairs CDR1 and 2 binding to MHC and allows weak binding to a self-p:MHC complex. Cells that undergo positive selection by recognition of a selfp: MHCI ligand lose CD4 and retain CD8, whereas cells that undergo positive selection by recognition of a self-p:MHCII ligand lose CD8 and retain CD4, in a process controlled by ThPOK and Runx3 transcription factors.8 In contrast, thymocytes that undergo negative selection either die by apoptosis or differentiate into Foxp3+ regulatory T cells.9
Recirculation
CD4+ and CD8+ T cells that complete positive selection exit the thymus and enter the secondary lymphoid organs (lymph nodes, spleen, and mucosal lymphoid organs). Just before leaving the thymus, these cells turn on the KLF2 transcription factor, which promotes expression of several gene products that control thymus exit, such as S1P1, and subsequent circulation through secondary lymphoid organs, such as CD62L and CC chemokine receptor (CCR) 7.10 T cells also express low levels of CD44 and CD45RO and high levels of CD45RB and CD45RA as they leave the thymus. Over the first 2 weeks after leaving the thymus in adults,
T cells increase the levels of expression of CD28 and the interleukin (IL)-7 receptor α chain, and become better able to proliferate and differentiate if confronted with the relevant foreign p:MHC ligand.11,12 After completing this maturation process, T cells express a CD44low CD45RO- CD45RBhigh CD45RA+ CCR7+ CD62L+ CD28high IL-7Rαhigh phenotype, which is used to identify naïve T cells.
T cells increase the levels of expression of CD28 and the interleukin (IL)-7 receptor α chain, and become better able to proliferate and differentiate if confronted with the relevant foreign p:MHC ligand.11,12 After completing this maturation process, T cells express a CD44low CD45RO- CD45RBhigh CD45RA+ CCR7+ CD62L+ CD28high IL-7Rαhigh phenotype, which is used to identify naïve T cells.
Naïve T cells spend their lives (T½ of 2 to 5 years in humans,13 and 50 to 100 days in mice14) recirculating through secondary lymphoid organs. Expression of trafficking molecules that bind ligands that are only expressed on the specialized blood vessels and sinuses of these organs explain this behavior. For example, the extravasation of naïve T cells through the high endothelial venules (HEVs) of lymph nodes and mucosal lymphoid organs depends on CD62L and CCR7, the ligands for which are only expressed on HEVs.15 After passing through the HEVs, naïve T cells enter the T-cell-rich paracortical areas of the lymph nodes and mucosal lymphoid organs and are constrained in these regions by sensing CCL19 and CCL21 with CCR7.16 Similarly, naïve T cells migrate from the marginal zone sinuses of the spleen into the T-cell-rich periarteriolar lymphoid sheathes (PALSs) via an unknown G-protein-coupled receptor-dependent mechanism. Naïve T cells again migrate within the PALSs through CCR7 sensing of CCL19 and CCL21. In all secondary lymphoid organs, naïve T cells are excluded from the B-cell-rich follicles due to lack of expression of CXC chemokine receptor (CXCR) 5, which binds to CXCL13 produced in follicles and guides cells to this location.
Survival
The T-cell areas within lymph nodes contain a network of thin collagen tubes called conduits, which carry lymphborne antigens and chemokines from the subcapsular sinus, where lymph enters the lymph nodes through afferent lymphatic vessels, to sinuses surrounding HEVs.17 The conduits are wrapped with fibroblastic reticular cells, which produce IL-7. Naïve T cells migrate along the conduits, placing themselves in a good position to receive IL-7, on which they depend for survival.18
To survive for a normal lifespan, naïve T cells must also receive TCR signals through weak recognition of self p:MHC ligands, perhaps the ones that caused the T cells to undergo positive selection in the thymus.1 Dendritic cells are probably the important antigen-presenting cell (APC) for this process because they are constantly in contact with T cells in the secondary lymphoid organs, and expression of MHCII molecules under the control of the dendritic cell-specific CD11c (Itgax) promoter is sufficient to maintain the survival of naïve CD4+ T cells. On the other hand, mice lacking dendritic cells by Itgax promoter-directed expression of diphtheria toxin have normal numbers of naïve T cells.19 This result does not rule out a role for dendritic cells in naïve T-cell maintenance, however, because it is possible that the naïve T cells die at a higher than normal rate without dendritic cells but are also produced at a higher rate. Studies on the turnover rate of naïve T cells in dendritic cell-deficient mice will be needed to address this question.
Self p:MHC ligand presentation by dendritic cells has different consequences for naïve T cells than foreign p:MHC ligand presentation. For example, self p:MHC ligand presentation results in only a subset of the signals that emanate from the TCR when bound by a high affinity foreign p:MHC ligand, including partial phosphorylation of the TCR-associated CD3-zeta chain.20 In addition, although signals through the TCR and IL-7 receptor are required for the survival of naïve T cells,21 these signals do not cause the T cells to proliferate in hosts containing normal numbers of T cells due to competition for IL-7. The Tsc122,23 and Foxp124 transcription factors, the second of which regulates IL-7Rα levels, enforce the quiescent survival of naïve T cells in this situation. In contrast, naïve T cells proliferate when the number of T cells is very low and IL-7 becomes more available, for example, early in life or after radiation or chemotherapy. This “homeostatic” proliferation also depends on IL-7 and low-affinity TCR recognition of self p:MHC complexes,21 but differs from proliferation in response to foreign p:MHC ligands by being independent of the CD28 costimulatory receptor.25 Thus, the same signaling events that cause naïve T cells to survive in interphase in T-cell-sufficient hosts cause these cells to proliferate in T-cell-deficient hosts, but using a program different from that engaged during the T-cell response to high affinity TCR ligands.
Survival and proliferation could both contribute to control of the number of naïve T cells in normal hosts. In adults, new naïve T cells are exported from the thymus into the secondary lymphoid organs that are already full of other T cells. Naïve T cells survive in interphase under these conditions. However, in neonates26 and perhaps the very aged, new naïve T cells enter relatively lymphopenic secondary lymphoid organs and undergo homeostatic proliferation to fill the space. Notably, this proliferation causes the T cells to lose many of the markers that define the naïve phenotype and express surface molecules that are characteristic of memory cells.21 Thus, although cells with the naïve phenotype dominate the preimmune repertoire, it can contain some cells with a memory cell phenotype.
Abundance
An adult mouse has about 108 αβ TCR+ T cells in the secondary lymphoid organs and another 5 × 106 in the blood.1 The naïve T-cell population is split about 60:40 in favor of CD4+ T cells over CD8+ T cells. About 70% of the cells in both subsets are naïve phenotype cells, at least in young mice housed under specific pathogen-free conditions. Thus, an adult mouse has about 7 × 107 naïve phenotype T cells in its body, which extrapolates to about 3 × 1011 naïve phenotype T cells in an adult human. Because the number of potential TCR amino acid sequences that could be produced by VDJ recombination (> 1015)27 is greater than the number of naïve cells in the body, it is possible that each naïve T cell has a different TCR. Thus, it is possible that each of the 7 × 107 naïve T cells in a mouse has a TCR capable of binding to one of 7 × 107 different foreign p:MHC ligands. This would mean that the frequency of naïve cells expressing TCRs specific for a single p:MHC ligand could be as low as 1/7 × 107 naïve T cells.
The actual number of naïve T cells specific for single foreign p:MHC-specific ligands has been measured by flow cytometry following labeling with fluorochrome-labeled pMHC tetramers and enrichment with anti-fluorochrome antibody-coated magnetic beads. C57BL/6 mice contain about 200 naïve CD4+ T cells specific for an immunogenic peptide called 2W bound to I-Ab but only about 20 cells specific for peptide 427-441 from the FliC protein of Salmonella typhimurium bound to I-Ab.28 Thus, the frequencies of cells capable of binding to these foreign p:MHCII ligands ranges from about 1:200,000 to 1:2,000,000 of the naïve phenotype CD4+ T cells in mice that were never exposed to these peptides. Similar analyses have been done for foreign p:MHCIspecific CD8+ murine T-cell populations.29,30,31 The number of naïve CD8+ T cells in C57BL/6 mice ranged from 15 lymphocytic choriomeningitis virus (LCMV) L338-346😀b-specific cells to 1,100 vaccinia virus B8R:Kb– specific cells per mouse.31
An important conclusion from these studies is that naïve populations specific for different foreign p:MHC ligands vary in size in a predictable fashion. There is evidence that size differences between foreign pMHC-specific populations in the preimmune repertoire are caused by negative selection.32 In other words, naïve foreign pMHC-specific populations can be small because their TCRs by chance bind with high affinity to self p:MHC complexes. Another possibility is that peptides that are recognized by larger naïve populations have structural features that are conducive to recognition by a more diverse set of TCRs (eg, amino acids with prominent side chains).32,33
Another important conclusion from these experiments is that the size of naïve p:MHC-specific populations can predict the magnitude of the effector cell response after certain forms of antigen administration. For example, the presence of about 200 2W:I-Ab– and 20 FliC:I-Ab-specific naïve CD4+ T cells correlates with the 100,000 2W:I-Ab– and 15,000 FliC:I-Ab-specific effector T cells induced by injection of the relevant peptides.28 Similarly, the size of naïve LCMV p:MHCI-specific T-cell populations plays a role the magnitude of the primary CD8+ T-cell response to the virus. LCMV infection of B6 mice activates CD8+ T cells specific for at least 28 different p:MHCI.31 However, about one-third of the total response is directed against three p:MHCI complexes. The feature of these dominant p:MHCI ligands that correlates best with their potent immunogenicity is the large size of their naïve populations.31 Therefore, although antigen abundance, efficiency of peptide generation by antigen processing, and MHC binding affinity are important factors in immunodominance,34 so is naïve T-cell population size.
GENERAL ASPECTS OF EFFECTOR T-CELL FORMATION
Unlike the presentation of low-affinity self p:MHC ligands that maintains the survival of naïve T cells in interphase under nonlymphopenic conditions, the presentation of highaffinity foreign p:MHC ligands induces the specific naïve T cells to produce lymphokines, proliferate, and differentiate into effector T cells that aid in elimination of the antigen from the body. These more dramatic biological effects occur because the responding T cells receive much stronger or more durable signals through the TCR upon recognition of high-affinity foreign p:MHC ligands than they do when recognizing low-affinity self p:MHC ligands.35 In addition, foreign antigens naturally enter the body during infection or tissue damage, which triggers dendritic cells and other innate immune cells to stabilize foreign p:MHC ligands and induce costimulatory receptors and cytokines.36,37 The nature of the signals from the TCR, costimulatory receptors, and cytokine receptors then influences the type of effector cells that naïve T cells differentiate into. These three signals and how they vary depending on the nature of the antigen will be described.
Dendritic Cells as Initiating Antigen-Presenting Cells
Arguably, the most important of the three signals is transduced by the TCR pursuant to bind to a foreign p:MHC ligand on an APC. It is clear from ablation experiments that dendritic cells are the only APCs capable of initiating the TCR signal in CD8+ and CD4+ T cells in the spleen.38 Dendritic cells are also important for initiating APCs for CD4+ T cells in lymph nodes, although other MHCII+ cells can do this job.38 It has also become clear that many dendritic cell subsets exist.38,39 A brief synopsis is presented here to give the reader a sense of which dendritic cell types function as APCs for different antigens. Refer to Chapter 16 on dendritic cells for more detailed information on their biology. Dendritic cells are defined in the mouse as cells that express the CD11c integrin, have large amounts of MHCI and MHCII molecules, and reside in or have the capacity to migrate to the T-cell zones of secondary lymphoid organs.40 The spleen and lymph nodes contain two types of dendritic cells that develop from monocyte-dendritic cell precursors in these organs. One type expresses the myeloid marker CD11b and is often referred to as the myeloid dendritic cell. These dendritic cells are found mainly in the red pulp or marginal zones of the spleen and outer T-cell-rich regions of the lymph nodes. A second type depends on the Batf3 transcription factor, expresses CD8α, and is referred to as the CD8α+ dendritic cell.41 These dendritic cells are located primarily in the PALSs of the spleen and the T-cell-rich regions of the lymph nodes, and are the major IL-12-producing cells in these locations. Myeloid and CD8α+ dendritic cells turnover rapidly with the latter population having only a 3-day half-life.
Another type of dendritic cell is called the plasmacytoid dendritic cell because of its morphology.42 These cells develop in the bone marrow and then seed the spleen and lymph nodes from the blood. Plasmacytoid dendritic cells in mice express low amounts of CD11c and the B220 and Gr-1 molecules normally expressed by B cells and granulocytes, and are the most potent producers of type 1 interferons (IFNs)43 when stimulated through pattern recognition receptors (PRRs).44 These cells therefore play a key role in inducing an antiviral state in many cell types. The lymph nodes contain several additional migratory CD11c+ dendritic populations that move to this location
from tissues through afferent lymphatic vessels.39 All lymph nodes contain CD11b+ dendritic cells that also express F4/80 and SIRPα, distinguishing them from myeloid dendritic cells and other CD103+ dendritic cells that often express an intracellular protein called langerin. In the case of the skin, these two types of dendritic cells migrate to lymph nodes from the dermis and are called dermal dendritic cells and dermal langerin-positive dendritic cells. The skin-draining lymph nodes also contain epidermal Langerhans cells, which express langerin and high levels of CD11b and EpCAM. Epidermal Langerhans cells are generated from a local radio-resistant precursor in the skin and are more long-lived than myeloid and CD8α+ dendritic cells.
from tissues through afferent lymphatic vessels.39 All lymph nodes contain CD11b+ dendritic cells that also express F4/80 and SIRPα, distinguishing them from myeloid dendritic cells and other CD103+ dendritic cells that often express an intracellular protein called langerin. In the case of the skin, these two types of dendritic cells migrate to lymph nodes from the dermis and are called dermal dendritic cells and dermal langerin-positive dendritic cells. The skin-draining lymph nodes also contain epidermal Langerhans cells, which express langerin and high levels of CD11b and EpCAM. Epidermal Langerhans cells are generated from a local radio-resistant precursor in the skin and are more long-lived than myeloid and CD8α+ dendritic cells.
The dendritic cell type that is most important for the presentation of p:MHC ligands depends on the nature of the antigen. Resident CD8α+ dendritic cells and CD103+ dendritic cell migrants are critical for p:MHCI ligand production from exogenous antigens because these are the only cells in the body that are capable of a process called cross presentation.41,45 These dendritic cells express receptors such as CD36 that mediate uptake of apoptotic cells. After taking up apoptotic or other extracellular material, these dendritic cells have the unique capacity to move proteins from the phagosome directly into the cytoplasm. Once in the cytosol, the translocated proteins can be cleaved by the proteosome into peptides, which are then pumped by the transporter associated with antigen processing into the endoplasmic reticulum where binding to MHCI occurs. Other cells of the body cannot translocate ingested proteins into the cytosol and thus are only capable of producing p:MHCI complexes from proteins that are translated in their cytosols (eg, their own proteins or proteins from cytosolic microbes that infect them). Thus, the cross presentation pathway is important for initiating the CD8+ T-cell response to viruses that do not infect dendritic cells but kill their host cells. In contrast, any dendritic cell including plasmacytoid dendritic cells can likely serve as an initiating APC for CD8+ T cells specific for MHCI-binding peptides derived from proteins from viruses that directly infect it.
Early activation of CD4+ T cells specific for p:MHCII ligands derived from soluble antigens can occur in two waves in lymph nodes.46 Soluble antigens (eg, toxins secreted by bacteria in a subcutaneous infection site) rapidly flow through lymphatic vessels to the draining lymph nodes and into the conduit network. Resident dendritic cells associate with the conduits, then take up the antigen, perhaps from small gaps between the fibroblastic reticular cells that encircle these tubes.47 These dendritic cells process the antigen, produce p:MHCII complexes, and display them for recognition by naïve CD4+ T cells. This process occurs within several hours of antigen deposition in the skin and results in CD69 induction and proliferation in the T cells.
The initial wave of p:MHCII presentation is followed by a second wave mediated by dermal dendritic cells that take up the antigen at the site of deposition and then migrate to the draining lymph node.46 These dendritic cells arrive in the T-cell areas 12 to 24 hours after antigen enters the tissue. p:MHCII presentation by these dendritic cells prolongs induction of the IL-2 receptor and is required for T-cell acquisition of the capacity to cause a later delayed-type hypersensitivity reaction.
Other types of antigens are accessed and processed by different dendritic cells. Given their location in the spleen, myeloid dendritic cells probably play a key role as initiating APCs for CD4+ T cells in the case of antigens that are present in the blood.48 Dendritic cells that migrate from the relevant tissue (eg, submucosal dendritic cells during vaginal infection are likely the only cells capable of producing p:MHCII ligands from microbes that are too large to enter the conduits.43 For unknown reasons, dermal Langerin-positive dendritic cells are the most important APCs for induction of contact hypersensitivity-causing CD8+ T cells by protein-modifying chemicals applied to the skin surface.49 Surprisingly, epidermal Langerhans cells are not required for contact hypersensitivity, although the sensitizing chemicals are applied to the skin surface. Rather, p:MHCII presentation by Langerhans cells inhibits the induction of delayed-type hypersensitivity by suppressing the priming of CD4+ Th1 cells and promoting the priming of Th17 cells.50
T-Cell Antigen Receptor Signaling
Once dendritic cells displaying foreign p:MHC ligands appear in the T-cell area of a secondary lymphoid organ, they can be recognized by naïve T cells expressing complementary TCRs. In vitro experiments have shown that highaffinity TCR ligation by p:MHC ligands causes the TCR to concentrate in a stable central supramolecular activating cluster (cSMAC) structure at the point of contact between the T cell and the APC.51 cSMAC formation is often preceded by the formation of TCR microclusters at the periphery of the T cell-APC contact zone, which are capable of the transducing signals.52 TCR clustering then activates protein tyrosine kinases such as lck, which stimulate signaling cascades that trigger protein kinase c theta, elevate intracellular calcium, convert ras into its active form, and activate the extracellular signal-regulated kinases (ERK1 and ERK2) and stress-activated protein kinases (jun kinase and p38 mitogen-activated protein kinase).53 These pathways culminate in the nuclear translocation and binding of transcription factors such as NFAT and NF-κB to deoxyribonucleic acid sequences that regulate lymphokine gene expression.54
Very little is known about early TCR signaling events in naïve T cells in vivo because the assays used to measure most of these events rely on cell lines and in vitro culture methods. Interestingly, in vivo imaging of naïve T cells showed rapid, p:MHC-dependent TCR internalization that was not contingent on prolonged contacts between T cells and APCs or cSMACs.55 These transient interactions must be sufficient for TCR signaling, however, because intracellular staining with antibodies that recognize the active forms of the c-jun transcription factor and the p38 mitogen-activated protein kinase showed that both of these molecules are phosphorylated in p:MHC-specific naïve T cells in the spleen within minutes of intravenous injection of the relevant peptide.56
This rapid response is likely explained by the fact that the majority of naïve T cells are constantly contacting dendritic cells in the T-cell areas.56
This rapid response is likely explained by the fact that the majority of naïve T cells are constantly contacting dendritic cells in the T-cell areas.56
Proliferation and Costimulation
Initial TCR binding to p:MHC ligands on dendritic cells causes naïve T cells to begin proliferating in vivo about 2 days later.57 In vitro experiments indicate that this cell division is driven by production of IL-258 and induction of the IL-2 receptor alpha chain (CD25).59 Surprisingly, however, p:MHC-driven proliferation of naïve T cells is minimally dependent on IL-2 in vivo.60,61 Therefore, other signals or growth factors must be capable of driving T-cell proliferation in vivo, although IL-2 may contribute. The role of IL-2 as a T-cell growth factor may be difficult to reveal because IL-2 is also required to maintain regulatory T cells62 and can promote death of activated T cells.60 Thus, a reduction in IL-2-dependent effector T-cell proliferation could be masked by a removal of regulatory T-cell-mediated suppression and activation-induced cell death. In addition, although IL-2 is not essential for initial p:MHCI ligand-driven T-cell proliferation, it does appear to be essential for memory cell differentiation.63
The initial proliferation of naïve T cells is followed by an exponential increase in the number of p:MHC-specific T cells over the next several days.64,65 Depending on the stimulus, the number of p:MHC-specific T cells reaches its highest level in the relevant secondary lymphoid organs, 5 to 7 days after antigen enters the body. As mentioned previously, naïve mice contain about 200 CD8+ T cells specific for a given p:MHC I complex. Because p:MHCI-specific CD8+ T cells can increase to 107 cells at the peak of the primary response,64,65 it follows that CD8+ T cells can expand 500,000-fold in vivo. Although naïve CD4+ T cells are also capable of dramatic clonal expansion when stimulated appropriately, their burst size appears to be less than CD8-positive T cells.64 For both CD4+ and CD8+ T cells, the amount of cell division is inversely proportional to the number of naïve precursors,14,66,67 indicating that in vivo proliferation is limited by competition between p:MHC-specific T cells.
In vivo T-cell proliferation is regulated by signals from the costimulatory CD28 molecule, which is triggered by binding to CD80 and CD86 on APCs.68 The proliferation of antigen-stimulated CD4+ or CD8+ T cells is greatly reduced in mice in which CD28 cannot interact with its ligands. CD40 ligand (CD154) deficiency also affects T-cell expansion,69 which may be related to the fact that CD40 signaling in APC induces CD80 and CD86.70 Although costimulatory signals enhance TCR-driven IL-2 production, the in vivo significance of this effect for T-cell proliferation is unclear, as described above. Although it has been proposed that CD28 acts by promoting TCR aggregation in the cSMAC,71 enhancing lymphokine messenger ribonucleic acid production72 and stability,73 and/or promoting T-cell survival by augmenting Bcl-XL production,74 the bulk of recent evidence indicates that it acts by NF-κB signaling.75 Members of the TNF receptor family, such as OX40, CD27, and 4-1BB are induced in T cells by CD28 signaling several days into the primary response.76,77 These molecules bind ligands of the TNF family on the surface of APC and transduce signals that sustain the proliferation or survival of p:MHC-stimulated T cells.
Enhancement of costimulatory signals may underlie the observation that in vivo T-cell proliferation is also influenced by inflammation at the time of initial p:MHC presentation. This effect is observed in the case of soluble antigens, where the magnitude of T-cell proliferation is several-fold greater if antigen is administered with a microbial substance78,79 containing a pathogen-associated molecular pattern43 such as lipopolysaccharide, which is recognized by a PRR such as toll-like receptor 4.80 PRR signaling stimulates tissue macrophages to produce tumor necrosis factor-α,81 which in turn stimulates dendritic cells to migrate from nonlymphoid tissues into the T-cell areas and express higher levels of CD80 and CD86.82 In addition, these signals result in a maturation process that changes the antigen processing and presentation potential of all dendritic cell types.83 When inflammation is not present, dendritic cells efficiently engulf extracellular fluid and produce p:MHC complexes from the ingested proteins. However, these p:MHC molecules turn over rapidly on the dendritic cell surface and are presented in the context of low amounts of CD80 and CD86. These factors are thought to be part of the explanation for why p:MHCII presentation by dendritic cells in uninflamed secondary lymphoid organs leads to poor T-cell priming and can result in tolerance.84 In contrast, inflammatory signals cause dendritic cells to reduce antigen uptake and processing, stabilize p:MHCII molecules, and induce expression of CD80 and CD86. Therefore, inflammatory signals enhance proliferation by driving more dendritic cells into the T-cell areas to present p:MHC ligands and by increasing the costimulatory capacity of these dendritic cells.
PRR signaling in dendritic cells or macrophages can also trigger the production of proinflammatory cytokines, which enhance effector cell proliferation. For example, IL-1 from several sources enhances the proliferation of CD4+ T cells through an early indirect effect on APCs,85 and a later more potent direct effect on the T cells themselves.86 PRR signaling causes CD8α+ dendritic cells to produce IL-1241 and plasmacytoid dendritic cells to produce type 1 IFNs,42 both of which enhance the proliferation of CD8+ effector T cells.87
EFFECTOR T-CELL DIFFERENTIATION
The proliferation of p:MHC-specific effector T cells during the primary response is linked with the acquisition of functions that affect the elimination of antigen such as microbicidal cytokine production and cytolysis. The functional properties that effector cells acquire are influenced by the presence of inflammatory cytokines and costimulatory ligands on APCs present at the time of initial p:MHC presentation in secondary lymphoid organs.
CD4+ T Cells
Many variations on this theme exist in the case of CD4+ T cells. Effector cells that are generated in the presence of IL-12, IL-4, IL-6 and TGF-β, or IL-6 and IL-21 become
IFN-γ-producing Th1 cells, IL-4-producing Th2 cells, IL-17-producing Th17 cells, or IL-21-producing follicular helper cells (Tfh), respectively. These effector T cells play specialized roles in the elimination of intracellular microbes, worms, and extracellular microbes. Polarization of Th cells is covered in detail elsewhere in this volume.
IFN-γ-producing Th1 cells, IL-4-producing Th2 cells, IL-17-producing Th17 cells, or IL-21-producing follicular helper cells (Tfh), respectively. These effector T cells play specialized roles in the elimination of intracellular microbes, worms, and extracellular microbes. Polarization of Th cells is covered in detail elsewhere in this volume.
Signaling through the IL-2 receptor plays an essential and early role in the formation of Th1, Th2, and Th17 effector cells. IL-2 receptor alpha chain (CD25) expression is induced in naïve T cells by TCR signaling, which then pairs with IL-2/IL-15 receptor β (CD122) and IL-2 receptor γ(CD132) chains, to produce the high-affinity IL-2 receptor.88 Under Th1 priming conditions, the IL-2-bound receptor recruits Jak1 and Jak3 that phosphorylate the STAT5 transcription factor, which enters the nucleus and enhances expression of the IL-12 receptor β2 chain and the T-bet and Blimp-1 transcription factors.89 IL-12 receptor signaling through STAT4 then enhances T-bet expression and enforces the Th1 fate,90 while Blimp-1 suppresses another transcription factor Bcl6 that is needed for other fates.91 Under Th2 priming conditions, IL-2 receptor signaling through STAT5 regulates the Th2 cytokine gene cluster and expression of the IL-4 receptor α-chain.89 In contrast, IL-2 receptor signaling inhibits Th17 differentiation by suppressing components of the IL-6 receptor.89
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree