Introduction
Peripheral arterial disease (PAD) occurs when blood flow reaching limbs is insufficient to fulfill the metabolic necessities of the tissue. This fact often comes from the presence of an occlusive arterial disease, the underlying disease process being atherosclerosis, which affects primarily, but not exclusively, the vascularization of the lower limbs.
Epidemiology
Risk Factors for PAD
The factors leading to PAD are mainly the same as those leading to atherosclerosis, a process that directly or indirectly accounts for 70% of all deaths in people older than 70 years. These factors are multiple: genetic factors, metabolic diseases, inflammatory diseases, lifestyle and local and systemic conditions of the vascular system. Among these conditions and diseases are the major risk factors: age, type 2 diabetes mellitus, hypertension, dyslipidaemia, smoking, physical inactivity and abdominal obesity, age being the only non-modifiable factor.
The prevalence of PAD varies depending upon the characteristics of the population and the criteria used to define its presence.1 There are two main methods to define PAD: the clinical one and the ankle-brachial index (ABI). The clinical method is based on the presence of the most classic symptom of PAD, namely intermittent claudication (IC), characterized by the presence of exertional calf pain that causes the patient to stop walking, resolves within 10 minutes of rest, does not resolve while the patient is walking, and does not begin at rest. The ankle-brachial index (ABI), a ratio of the systolic blood pressures in the lower and upper extremities obtained by Doppler, is the most widely used diagnostic test for detecting PAD. Among the participants in the Cardiovascular Health Study, an epidemiologic evaluation of 5084 community-dwelling men and women 65 years or older, the prevalence of PAD as defined by ABI was 12%, whereas only 2% of participants had IC.2 The PARTNERS (PAD Awareness, Risk, and Treatment: New Resources for Survival) study, with 6979 men and women from primary care (aged 50–69 years with history of diabetes mellitus or cigarette smoking and 70 years or older), which also used the ABI as the diagnostic criteria, found a prevalence of 29%. Only 11% of these patients with PAD had IC.3 The majority of the men and women diagnosed with PAD based on the ABI do not have classic symptoms of IC. The prevalence of classic symptoms (Table 43.1) varies from ∼10–30% in patients with PAD based on the ABI value.
Table 43.1 Stages and symptoms of PAD.
| Fontaine’s stages | |
| Stage | Clinical |
| I | Asymptomatic |
| IIa | Mild claudication (more than 150 metres) |
| IIb | Moderate–severe claudication (less than 150 metres) |
| III | Ischaemic rest pain |
| IV | Ulceration or gangrene |
Age is the main risk factor for PAD. In fact, its prevalence increases dramatically with age. The Cardiovascular Health Study found a prevalence of PAD around 30% in men older than 85 years while it was lower than 10% in men ranging from 65–69 years old. The prevalence of PAD in women aged from 65–69 years old was lower than 5%, but exceeded 35% in those aged 85 or older. This association between older age and higher prevalence of PAD was also observed in both women and men with a history of heart disease and stroke.2 Data from 2174 subjects aged 40 years and older from The National Health and Nutrition Examination Survey 1999–2000 show a PAD prevalence of 4.3% (95% CI, 3.1–5.5%) in the whole sample, but among those 70 years or over the prevalence was 14.5% (95% CI, 10.8–18.2%).4
Age is not only the most important non-modifiable factor which increases the risk of PAD two- to threefold. In addition, the pattern of the disease changes according to age: aorto-iliac disease occurs usually in younger subjects and is more rapidly progressive than distal disease.
Other risk factors besides age are smoking, diabetes, hypertension, dislipemia and black race. In the National Health and Nutrition Examination Survey 1999–2000,4 more than 60% of individuals with PAD had hypercholesterolaemia, 74% were hypertensive, 26% had diabetes and 33% were current smokers. Approximately 95% had, at least, one of these cardiovascular risk factors and 72% had two or more. When adjusted by age and gender, current smoking (OR 4.46; 95% CI, 2.25–8.84), diabetes (OR 2.71; 95% CI, 1.03–7.12), black race/ethnicity (OR 2.83; 95% CI, 1.48–5.42), low kidney function (OR 2.00; 95% CI, 1.08–3.70), hypertension (OR 1.75; 95% CI, 0.97–3.13) and hypercholesterolaemia (OR 1.68; 95% CI, 1.09–2.57) remained positively associated with prevalent PAD. After adjustment for smoking status, BMI, hypertension, hypercholesterolemia, diabetes and glomerular filtration rate, the smoking status (OR 4.23; 95% CI, 1.95–9.17), black ethnicity (OR 2.39; 95% CI, 1.11–5.12), diabetes (OR 2.08; 95% CI, 1.08–4.28) and low kidney function (OR 2.17; 95% CI, 1.10–4.30) remained significantly associated with the presence of PAD.
Smoking is a major and modifiable risk factor for PAD. Smoking not only predisposes to develop PAD but also increases its severity and affects the prognosis of revascularization interventions. In patients who smoke, PAD affects predominantly proximal arteries, especially the aorta and iliac arteries.5
The prevalence of PAD is approximately twice as high for individuals with diagnosed diabetes. Diabetes mellitus is not only a qualitative risk factor but also a quantitative one. In fact, glycaemic control is one of the strongest risk factors of illness: a positive, graded, and independent association between HbA1c and PAD risk has been described in adult people with diabetes.6 This association is stronger for clinical (symptomatic) PAD, where manifestations may be related to the existence of microvascular disease, than for asymptomatic PAD. The glycaemic control is also associated with the worst consequence of this disease: amputation. In this regard, amputation is 10 times more frequent in diabetic than in non-diabetic patients. This suggests that efforts to improve glycaemic control in persons with diabetes may substantially reduce the risk of PAD. However, the efficacy of an intensive glycaemic control (reaching HbA1c values lower than 7%) to prevent PAD as compared with other less stringent therapeutic goals (∼8%) is not established and, in fact, may have adverse consequences. Another important risk factor to develop PAD in diabetic patients is the presence of albuminuria, whatever its magnitude. Diabetes causes predominantly distal occlusive disease, including the arteries of the calves and feet.5
Although arterial hypertension contributes to the development of PAD to a lesser extent than either of the two previously mentioned factors, it is also a risk factor that must be controlled. There are limited data regarding the association between hypertension and PAD according to lesion localization.5
Black ethnicity is a strong and independent risk factor for PAD. At first, some epidemiological studies suggested a differential relationship between risk factors and prevalence of lower peripheral disease in people from different ethnicities. However, data from recent studies cannot explain the excess risk of PAD in black people by a higher prevalence of diabetes or hypertension, increased BMI or higher levels of some of the new cardiovascular risk factors (interleukin-6, fibrinogen, D-dimer, homocysteine). This suggests that unknown factors may account for the residual differences. In clinical series, PAD in blacks is associated with poorer prognosis after revascularization because of the greater presence of distal lesions involved.5
The Cardiovascular Health Study assessed the incidence rate of abnormal ABI over time in a community population (5888 participants, men and women 65 years and older, without PAD) and tried to identify what cardiovascular risk factors were predictors of ABI decline. ABI decline occurred in 9.5% of this elderly cohort over six years and was associated with modifiable vascular disease risk factors: current cigarette use (OR 1.74; 95% CI, 1.02–2.96), hypertension (OR 1.64; 95% CI, 1.18–2.28), diabetes (OR 1.77; 95% CI, 1.14–2.76), higher low-density lipoprotein cholesterol (LDL-C) level (OR 1.60; 95% CI, 1.03–2.51), and lipid-lowering drug use (OR 1.74; 95% CI, 1.05–2.89).7
Consequences of PAD
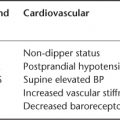
Peripheral arterial disease increases the risk of total mortality. Among these patients, more severe disease, as measured by the ABI, is associated with increased mortality. For example, mortality is higher in patients with an ABI less than 0.50 than in those with an ABI between 0.50 and 0.90. Traditionally, an ABI greater than 1.40 has been considered of little diagnostic value because it was believed that it indicates the presence of non-compressible lower extremity arteries. However, non-compressible arteries may be the manifestation of the presence of calcification of the media layer of the artery, a common condition in patients with diabetes and chronic kidney disease that is associated with increased mortality. Individuals with ABI values greater than 1.40 have a higher prevalence of intermittent claudication and atypical leg symptoms, suggesting an increased prevalence of PAD among these individuals. In fact, an ABI index higher than 1.40 is associated with an increased mortality. In this regard, The Cardiovascular Health Study found an increase in total mortality in patients with basal ABI greater than 1.40 compared with a normal ABI. The magnitude of this increased mortality risk in people with an ABI greater than 1.40 was similar to that observed in people with an ABI less than 0.90. This suggests that across the spectrum of ABI values, the association between the ABI and mortality appears to be ‘U’ shaped.1
Peripheral arterial disease is a strong predictor of subsequent cardiovascular morbidity and mortality. Associations between PAD and cardiovascular mortality are independent of age, BMI, cigarette smoking, LDL-C, HDL-C, blood pressure, fasting glucose levels and history of angina, myocardial infarction, stroke or other heart problems. This association has been observed in multiple populations (clinical and community settings, general population and special groups like elderly or diabetic patients, subjects with and without classic intermittent claudication, etc). It has been reported over relatively short-term (3–4 years) and long-term (10 years) follow-up. Like the association between PAD and total mortality, the Strong Heart Study found a higher cardiovascular mortality risk if ABI was less than 0.90 or greater than 1.40, describing a ‘U’ shaped line too. PAD is associated with prevalent cardiovascular disease and adverse cardiovascular disease risk factor profiles. Prospective studies using the ABI show in people with a low ABI, a higher incidence of stroke (mainly in the elderly), fatal and non-fatal coronary disease, heart failure and a poor prognosis in all forms of cardiovascular disease. Because of that, identifying persons at both ends of the ABI distribution may be a useful method for cardiovascular risk stratification and may also be an indication for a comprehensive management of cardiovascular risk factors.8 However, in the cardiovascular risk stratification, it is not just the grade of PAD that is important. The progression of PAD, measured as the changes in ABI over time, can add information in this evaluation of the cardiovascular risk. Criqui et al. demonstrated that progressive PAD (ABI decline >0.15) was significantly and independently associated with an increased cardiovascular risk.9
In addition to the classical implications of PAD on the cardiovascular disease, in the elderly PAD also plays a crucial role in determining an impaired functional status. Specifically, elders with PAD have lower physical activity levels, slower walking speed, poorer balance and poorer walking endurance. Data obtained from 1798 participants 60 years and older who participated in the population-based National Health and Nutrition Examination Survey,10 showed several relevant findings about the relationship between PAD and physical function. Participants were asked about their dependence in performing several tasks (basic activities of daily living, instrumental activities, social activities, general physical activities and capacity to walk one-quarter mile and to walk up 10 steps). Trained health technicians measured ABI, maximal right leg force and gait speed. There were differences in both self-reported functional dependence and performance-based physical measures. Gardner and Montgomery11 compared two groups of subjects 65 years and older, the first group with symptomatic PAD and the second one without illness. PAD patients had 28% shorter unipedal stance time, 86% higher prevalence of ambulatory stumbling and unsteadiness, and 73% higher prevalence of falling than non-PAD patients. Instability in patients with PAD was exacerbated in those with a worse ambulatory function and lower levels of physical activity. These findings remind us of the old scheme about the theoretical model of the pathway to late-life dependence proposed by Verbrugge.12 He defined four related but different categories that represent the causal connection between pathology and actual dependence. These are: ‘active pathology’ (such as PAD), ‘impairment’ (such as low leg force or low ABI), ‘functional limitation’ (such as slow gait speed) and ‘disability’ (such as dependence in activity daily living). As a consequence, for a complete evaluation of an elderly patient with suspected PAD, performance-based physical measures (gait speed, strength, unipodal time, full tandem stance time, etc.) must be included.
Gardner et al.13 followed 43 men limited by intermittent claudication (mean age, 69 years ±7) during 18 months to understand the natural evolution of physical performance in patients with PAD. They experienced a decline in six-minute walk performance, monitored and self-reported physical activity, physical performance, measured and self-reported stability and calf blood flow despite no change in ABI. While it may be assumed that all these changes could cause an impaired balance and a higher prevalence of falling, putting PAD patients at higher risk for serious injury, restricted physical activity, higher cost and more frequent hospitalizations, higher nursing home admissions and greater mortality, the actual clinical consequences and prognostic significance of functional impairment in persons with PAD was unknown until the study by McDermott et al.14 They carried out a prospective observational study with 638 participants followed for a median of 50 months. Among PAD participants, the risk of mobility loss, measured as the hazard ratio (HR), in the lowest versus the two highest quartiles of baseline performance for the six-minute walk test was 9.65 (95% CI, 3.35–27.77, p < 0.001). For the Short Physical Performance Battery the adjusted HR was 12.84 (95% CI, 4.64–35.55, p < 0.001). They concluded that differences in the rate of mobility loss between PAD persons and healthy subjects appear to be primarily related to poorer lower-extremity performance at baseline. The measures of lower extremity performance predict risk of mobility lost and risk of mortality;15 they are simple and easy to perform in the office. So, they can be used to identify PAD persons at highest risk of mobility loss.
The ‘vulnerability’ of these patients could suggest a possible relationship between PAD and frailty. In The Cardiovascular Health Study the ABI was inversely related to frailty status, in elderly people with or without clinical cardiovascular disease.16 The prevalence of progressively lower levels of ABI increased in each level of frailty (non-frail, pre-frail, frail). These data, along with the other results in the CHS, suggest that cardiovascular disease appears to be an important, but not sole, contributor to frailty.
Finally, the association between PAD and other apparently unlinked diseases must be emphasized. This is the case for depression and dementia. An increased prevalence of depression in patients with PAD has been detected. One explanation could be that the functional impairment accompanying PAD affects QOL and may lead to depressive symptoms.
ABI is associated with the incidence of total dementia, vascular dementia and Alzheimer’s disease,17 as is shown in the data from the Honolulu-Asia Aging Study, a prospective community-based study of Japanese American men, older than 70 years at baseline. The analysis included 2588 men who were free of dementia at the first assessment, had an ABI measurement, and were examined up to twice more for dementia. After adjustment for education, year of birth, high blood pressure, BMI, diabetes mellitus, cholesterol concentration, smoking status, alcohol consumption and apolipoprotein E4 allele, a low ABI was associated with an increased risk of dementia (HR, 1.66; 95% CI, 1.16–2.37) and vascular dementia (HR, 2.25; 95% CI, 1.07–4.73). ABI was weakly associated with Alzheimer’s disease (HR, 1.57; 95% CI, 0.98–2.53), particularly in the apolipoprotein E4 carriers (HR, 1.43; 95% CI, 1.02–1.96).
Despite what is claimed, the public is poorly informed about PAD. Current data suggest that PAD detection and treatment are lower than other forms of atherosclerotic disease, although there is evidence to support a prognostic significance and an impact on QOL comparable to other forms of cardiovascular disease.18 As a consequence it is essential to make an active search of the disease. The Comprehensive Geriatric Assessment (including performance-based measures) plus ABI should be used to stratify the cardiovascular risk and to detect and prioritize the patients in whom an aggressive approach is needed.
Pathophysiology
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree