9 Perioperative Management
Optimizing the perioperative management of a patient with a brain tumor can have a profound effect on their surgical outcome. Attention to important details leads to better decisions and optimal patient recovery. This chapter addresses perioperative issues that are fundamental for appropriately managing patients undergoing surgical intervention for brain tumors. Although these principles can be generalized to every surgical patient, the importance of encompassing the needs of the individual patient should be emphasized.
 Preoperative Considerations
Preoperative Considerations
History and Physical Examination
The three most important initial steps in evaluating a patient with a brain tumor are to obtain a comprehensive history of the patient’s illness, carefully examine the patient, and obtain the appropriate imaging studies. These elements compose the foundation on which the perioperative assessment is established.
A detailed history can provide important clues regarding the likely underlying pathology, and, coupled with a thorough neurologic examination, can often reveal the lesion’s location. Brain tumors come to clinical attention when they have reached a critical size or have caused sufficient brain swelling to result in headache, seizure, change in functional capacity (e.g., paresis, vision changes), or cognitive impairment.1 The timing with which these problems arise is often inversely related to the aggressiveness of the tumor, such that the shorter the presentation and more rapid the onset of signs and symptoms, the more aggressive the underlying tumor pathology. Patients with malignant tumors, such as glioblastoma multiforme (GBM) or brain metastases, can often present with worsening focal neurologic deficits, occurring over days to weeks, or with the acute onset of seizure. In contrast, indolent lesions, such as low-grade glioma or meningioma, can remain clinically silent for years until they reach a critical size, at which time small changes in tumor volume or characteristics (e.g., hemorrhage) can generate neurologic symptoms.1–3
Because the temporal evolution of the history gives some indication of the anticipated growth rate of the tumor, it is important to inquire about signs and symptoms that may be considered by the patient to be unremarkable or unrelated. Partial epilepsy, for instance, is a common undetected sign of low-grade intrinsic brain tumors and can be unrecognized, often with a subtle history of progressively worsening difficulties at school or work, as well as unexplained learning disabilities. Such changes in function may be misinterpreted as the result of stress or inattention. In addition, the rapidity with which signs and symptoms evolve can also serve as a good indicator of how quickly intervention should be pursued, with more acute changes in functional capacity or altered consciousness necessitating a more urgent surgical intervention.
Pearl
• A family history, when appropriate, can be of particular importance to identify families with germline mutations that predispose family members to cancer.
Although most neurosurgeons are comfortable with interpreting the results of a routine neurologic examination, this may prove to be inadequate to illustrate the impact of the tumor on cognitive abilities. Executive function can be further quantitatively assessed through psychometric testing.4 Patients with tumors in the dominant mesial temporal lobe, for instance, are at risk for profound memory impairment with surgery, and preoperative memory testing can help determine the relative risk. In those patients who have memory loss of greater than two standard deviations from the mean, surgery typically does not result in a clinically significant further decline.5 Although most right-handed patients maintain dominance for language and memory in the left hemisphere, long-standing lesions (e.g., low-grade glioma) as well as prior cortical injury can result in reorganization of function that is shifted to, or shared with, the right hemisphere. Such patients may benefit from investigation with preoperative functional studies to determine the localization of language and memory.
A family history can also be of importance. Immediate relatives with a history of brain tumors can raise the possibility of an inheritable predisposing condition (e.g., germline mutations) such as neurofibromatosis, von Hippel–Lindau disease, Turcot’s syndrome, or Li-Fraumeni syndrome. Patients are often unaware of such familial risks, and a careful review of the family’s medical history and a dedicated physical examination, including a search for café-au-lait macules in potential neurofibromatosis patients, can facilitate more frequent and thorough monitoring of the patient and family members.
 Preoperative Imaging
Preoperative Imaging
Tumor location is one of the most important findings to consider in deciding on the type of surgical intervention, such as biopsy versus resection. The proximity of the tumor to motor, somatosensory, language, and visual areas, as well as to critical neurovascular structures, can help determine the relative risk of surgery (Fig. 9.1). The size of the lesion and its extension into the surrounding brain can also provide an estimate of the probability of achieving the surgical goals.
The most commonly used imaging modalities for evaluating a patient with a brain tumor are computed tomography (CT) and magnetic resonance imaging (MRI). MRI is superior for intrinsic tumors as it not only provides a high-resolution definition of normal anatomy but also better delineates tumor extension, invasion and displacement of normal structures. CT can be useful in cases with bone erosion or hyperostosis. Evaluating images in multiple planes aids in a three-dimensional understanding of the tumor and is crucial for surgical planning.
Specific imaging findings can be useful for establishing a probable diagnosis and offer insight into the likely pathology. Low-grade gliomas are typically hypodense on CT and hyperintense on T2-weighted MRI—the majority being without appreciable contrast enhancement. Alternatively, most GBMs have a central nonenhancing region of necrosis surrounded by a ring of enhancement composed of tumor and neovascularity, along with a region of infiltrative tumor cells that can best be appreciated as abnormal T2-weighted signal extending into the surrounding brain. Contrast enhancement signifies an impaired blood–brain barrier with heterogeneous and homogeneous patterns typically displayed in high-grade tumors and meningiomas, respectively. The acquisition of contrast enhancement in a previously nonenhancing glioma is an indication that the tumor has evolved into a more anaplastic lesion.
The presence of contrast enhancement, however, lacks specificity for malignancy. Noninfiltrative low-grade gliomas, such as pilocytic astrocytoma and pleomorphic xanthoastrocytoma (PXA), display nodular enhancement. In addition, up to 25% of malignant gliomas may have faint or no detectable enhancement, whereas 30% of diffuse low-grade gliomas can demonstrate contrast enhancement.6,7 Thus, interpretation of imaging must be made in the context of clinical information. Nonetheless, the risk of anaplasia in nonenhancing tumors increases with age.8
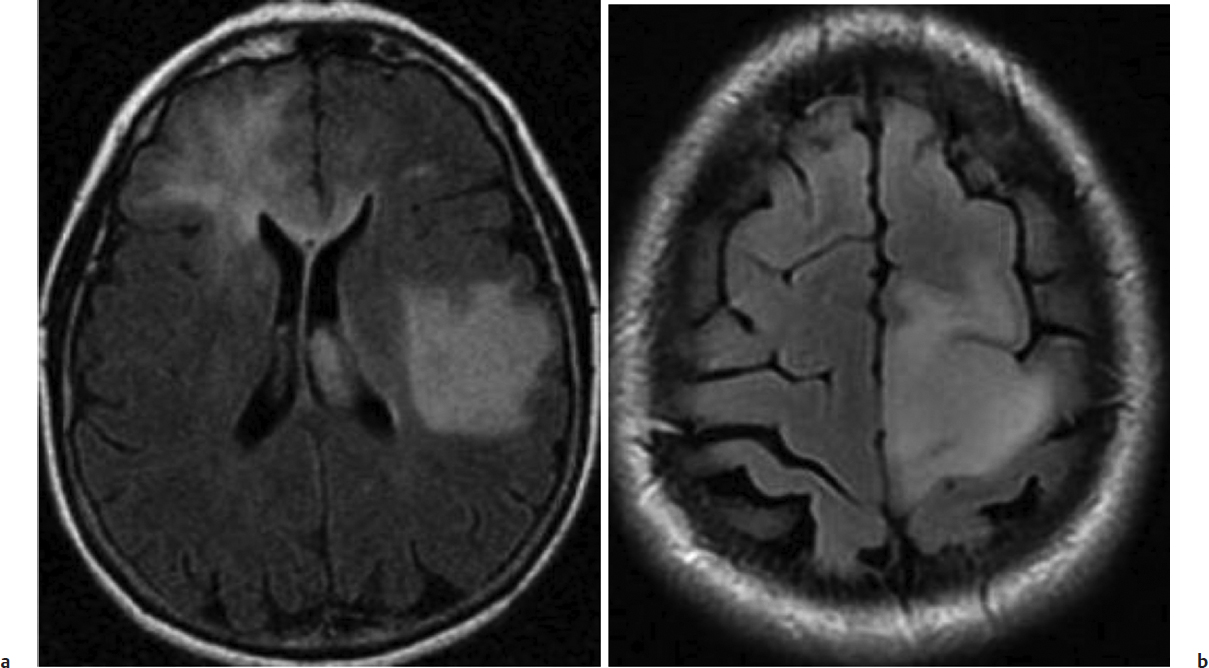
Fig. 9.1a,b (a) Axial fluid-attenuated inversion recovery (FLAIR) image of diffuse tumor infiltration into the left posterior frontal lobe and the right frontal lobe with involvement of the corpus callosum. These findings indicate a diffuse glioma. (b) Axial FLAIR image demonstrating a glioma within the left motor cortex. Both images show tumors that cannot be removed without serious neurologic sequelae and are best managed with a biopsy.
• The absence of contrast enhancement within a glioma on MRI does not exclude the possibility of a high-grade tumor. Restricted diffusion on DWI can indicate a highly cellular tumor or particularly cellular area of a tumor that can help direct biopsy.
Imaging correlates of molecular tumor characteristics have been recognized. Oligodendrogliomas with deletions on chromosome 1p and 19q are usually hypointense compared with white matter and have indistinct borders on T1-weighted MRI, whereas tumors that lack such molecular features more commonly are sharply demarcated from surrounding brain. In addition, co-deleted oligodendrogliomas are characterized by signal heterogeneity on T1- and T2-weighted images with focal paramagnetic susceptibility effects likely related to calcium deposition within the tumor. Anaplastic tumors lacking 1p/19q deletions are characterized by ring enhancement.9,10 Magnetic resonance imaging criteria have also been established for high-grade gliomas featuring overexpression of the epidermal growth factor receptor (EGFR).11
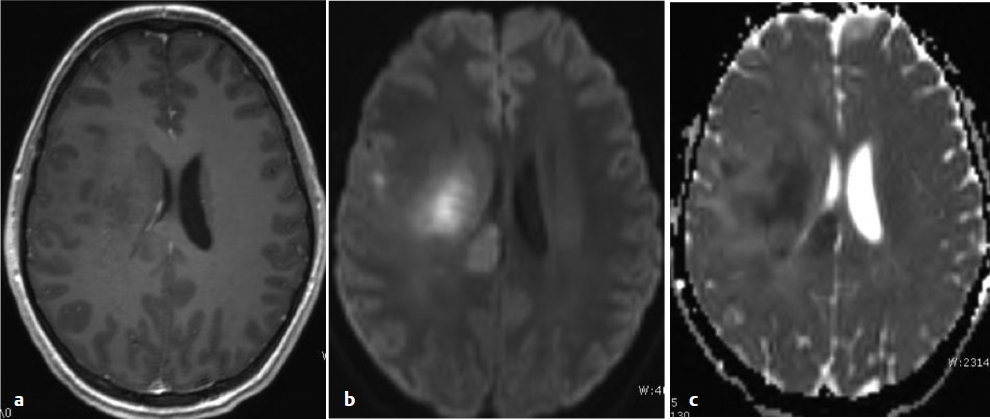
Diffusion-weighted imaging (DWI) delineates tissue characteristics based on the molecular motion of protons and can be quite useful. In a tumor of dense cellularity, such as GBM and lymphoma, the intracellular compartment is increased relative to the extracellular, and the diffusion signal is increased compared with normal brain. As a result, the signal of these tumors is often increased on DWI and correspondingly decreased on apparent diffusion coefficient (ADC) maps (Fig. 9.2). Diffusion-weighted imaging not only facilitates differential diagnosis of neoplasms and other structural lesions but also may be useful to identify a focus of increased cellularity and thus a favorable biopsy target, especially within a non-enhancing tumor. Areas of restricted diffusion in a nonenhancing low-grade glioma may reflect an early imaging sign of high-grade transformation.12 Abscesses can lead to restricted diffusion, which once again underscores the need for considering the entire clinical picture.13
Functional Studies
Functional MRI (fMRI) can prove valuable for the surgical management of tumors located in or near eloquent cortical and subcortical anatomy.14 The technique relies on focal changes in blood flow caused by a repetitive stereotypic activity to localize regions that subserve a specific function. Functional MRI is most often used to localize the primary motor and somatosensory cortex. Speech and language function are also commonly mapped with fMRI; however, the precision of this technique and the variability and complexity in localizing language function makes this less reliable. Tumors can invade or displace these critical regions, and functional anatomic imaging can help define anatomic relationships that direct surgical strategy.
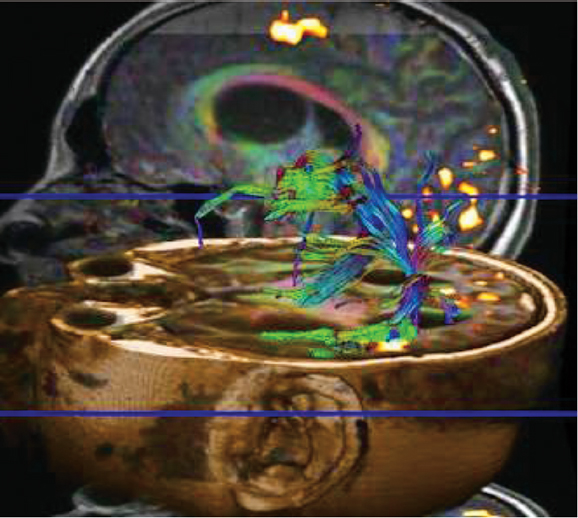
Diffusion tensor imaging (DTI) takes advantage of preferential diffusion of water molecules in the direction of fiber bundles.15 The distribution of major vectors in white matter (tractography) is commonly displayed using color-based maps (Fig. 9.3). Displacement or invasion of these fiber tracts can be identified in patients with mass lesions in the brain, and this information can also be critical in avoiding major neurologic complications during tumor surgery.
Fig. 9.2a–c (a) Axial T1-weighted MRI demonstrating a nonenhancing tumor in the right centrum. (b) Diffusion-weighted imaging of the same tumor showing areas of restricted diffusion (high cellularity), with (c) an apparent diffusion coefficient map of the same tumor, demonstrating dark regions matching the areas of restricted diffusion. These findings are suggestive of early anaplastic transformation of a low-grade glioma.
Other Imaging Modalities
Proton magnetic resonance spectroscopy imaging (1H-MRSI) has been utilized to map the infiltration of tumor cells into the brain by examining the relative concentration of choline (Cho), N-acetyl aspartate (NAA), and creatine (Cr).16–18 The profile of each of these major metabolites in tumor tissue is distinct from the relative amounts found in normal brain. By examining their relative concentrations across a volume of tissue, metabolic maps of the extent of the tumor can be produced. The presence of lactate peaks on MRSI, for instance, often indicates anaerobic metabolism found in high-grade tumors. When the anatomic MRI presents an ambiguous lesion, MRSI can be useful for predicting the diagnosis of a glioma. The distribution of MRSI-identified tumor infiltration often extends beyond the volume identified by routine MRI.
p-[123I]iodo-l-phenylalanine accumulates in tumors when imaged with single photon emission computed tomography (SPECT) and can help distinguish gliomas from nonneoplastic lesions.19
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree