PATHOPHYSIOLOGY OF TESTICULAR DYSFUNCTION
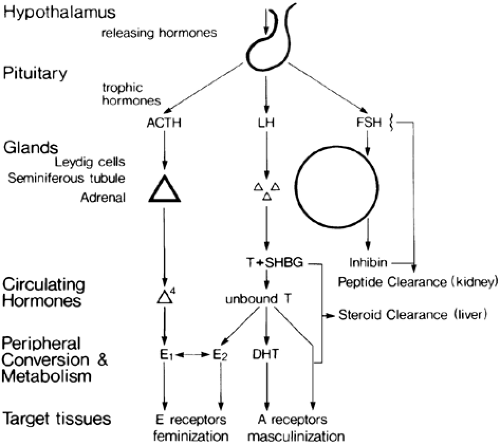
Control of the pituitary-testicular axis is discussed in detail in Chapter 16, Chapter 113 and Chapter 114. Figure 116-1 is a schematic outline to aid understanding of the changes that occur with hypogonadism. The pituitary-testicular axis displays a limited range of responses to stress. Generally, acute stresses are associated with gonadotropin suppression and secondary testicular failure, and chronic stresses are associated with primary testicular failure and elevated gonadotropin levels. Both patterns of abnormal testicular function appear to be reversible. Studies performed in
patients after kidney or liver transplantation show that recovery of testicular function is possible.3,4,5,6 and 7
patients after kidney or liver transplantation show that recovery of testicular function is possible.3,4,5,6 and 7
Most studies of testicular function in various systemic diseases have been small and cross-sectional, and it sometimes is difficult to know whether the hormone patterns are stable or evolving. For example, low testosterone and high luteinizing hormone (LH) levels may indicate either primary Leydig cell dysfunction or a recovery phase after gonadotropin suppression.
CLINICAL FEATURES OF TESTICULAR FAILURE
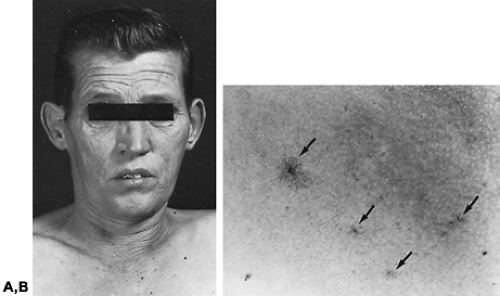
Testicular failure with an onset before the end of childhood prevents the full development of masculine features; the genitalia remain infantile in appearance, and secondary sex characteristics, such as deepening of the voice and pubic, beard, and body hair, do not develop. Epiphyseal fusion is delayed, and long bone growth is proportionately greater than that of the axial skeleton, causing eunuchoidal proportions (see Chap. 115). Deterioration of testicular function after normal puberty decreases sperm and testosterone production, manifesting clinically as the loss of libido, impotence, infertility, reduced hair growth, atrophy of the prostate and other accessory sex organs, and a reduced semen volume if ejaculation is possible (Fig. 116-2). There may be dryness of the skin from reduced sebum production and wrinkling of the skin on the face. Atrophy and depigmentation of the genital skin and hot flushes also may occur, but the latter are less marked symptoms of gonadal failure in men than they are in women. Other features possibly attributable to androgen deficiency include lethargy and weakness with loss of muscle bulk and increased subcutaneous fat on the trunk. Anemia and osteoporosis may result from prolonged androgen deficiency. Gynecomastia commonly accompanies hypogonadism of many causes (see Chap. 120). Spider nevi may be seen with testicular failure unassociated with liver disease or alcoholism, although rarely in large numbers8 (see Fig. 116-2).
DEVELOPMENT OF THE CLINICAL FEATURES OF TESTICULAR FAILURE
SUPPRESSION OF GONADOTROPIN SECRETION
Severe, acute illnesses are associated with low gonadotropin levels.1,9 Presumably, the output of gonadotropin-releasing hormone (GnRH) is reduced by a central mechanism9 (see Fig. 116-1). It is suggested that there is a common underlying mechanism termed ontogenic regression similar to seasonal regression of reproductive function in animals, which acts to conserve the individual under adverse circumstances.5 However, many mechanisms may contribute, including the effects of fever, drugs, cytokines, or stress hormones either directly on the testes or indirectly on the hypothalamus and pituitary.1,5,6,7,8,9,10,11,12,13,14,15,16 and 17 Primary and secondary hypogonadism may alternate or may be superimposed. For example, a suppression of previously high gonadotropin levels occurs during the terminal stages of cirrhosis with the onset of hepatic coma.8 Conversely, gonadotropin levels, which are suppressed during severe starvation, may be elevated with refeeding, causing a transient excess of estrogen production and refeeding gynecomastia. The mechanism of this
condition may be similar to that of the physiologic gynecomastia of puberty.18,19
condition may be similar to that of the physiologic gynecomastia of puberty.18,19
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree