Fig. 10.1
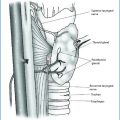
a A case of intrathyroidal parathyroid adenoma (hematoxilin and eosin, original magnification 40×). b Negative for thyroglobulin (immunoperoxidase, original magnification 40×). c Positive for chromogranin A and GATA-3 (immunoperoxidase, original magnification 40×; inset: original magnification 200×)
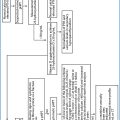
Diagnosis of malignancy. To define the type of parathyroid neoplasia, numerous ancillary immunohistochemical markers of cell cycle and proliferation have been evaluated, including Ki-67, cyclin D1, p27kip1, Rb, bcl2, mdm2, calcium-sensing receptor and galectin-3. Although carcinomas have a higher Ki-67 proliferation index than adenomas, the overlap in proliferative fractions between these tumor types has limited the utility of Ki-67, particularly in equivocal cases. p27 is an inhibitor of cell cycle dependent kinases and was demonstrated to have a threefold decrease in carcinomas compared to adenomas. Therefore, it was suggested that low p27 and high Ki-67 may be useful in the differential diagnosis of carcinoma from adenomas. On a tissue microar-ray-based approach, the phenotype p27(+)/bcl2(+)/Ki-67(-)/mdm2(+) was reported as indicative of nonmalignant neoplasms [5]. Loss of nuclear parafi-bromin immunostaining, due to inactivating mutations of HRPT2 gene (see Section 10.1.3.3), may aid the diagnosis of parathyroid carcinoma, being found both in parathyroid carcinomas of patients with hyperparathyroidism-jaw tumor syndrome (HPT-JT) and in about 70% of sporadic parathyroid carcinomas [8]. Moreover, negative parafibromin staining predicts the malignant nature (and behavior) of so-called “atypical adenomas” [9].
10.1.3.3 Genetics of Parathyroid Neoplasms
Comparative genomic hybridization (CGH) studies have allowed the identification of putative tumor suppressor genes and oncogenes peculiarly modified both in adenoma and in carcinoma.
Adenoma. Loss of the long arm of chromosome 11q, the site of the tumor suppressor gene MEN1, is the most frequent genetic abnormality in parathyroid adenomas, as loss of heterozygosity (LOH) of 11q13 has been demonstrated in 25–40% of adenomas with approximately 50% of these cases being associated with somatic homozygous mutations of the MEN1 gene. At the same locus 11q13 also the cyclin D1 (CCND1)/PRAD1 oncogene is mapped (for parathyroid adenoma), which frequently harbors rearrangements leading to an overexpression of cyclin D1 protein in up to 40% of cases [10].
Carcinoma. CGH analyses have demonstrated losses of 1p and 13q in more than 40% of cases of parathyroid carcinoma. Interestingly, loss of 11q13, the most common abnormality of adenomas, was undetectable in carcinomas, suggesting that adenomas developing along the MEN1 pathway have minimal potential for progression into carcinomas [10]. Although LOH of RB and BRCA2 genes, both on chromosome 13q, have been frequently described, their role remained controversial, as the direct sequencing of parathyroid carcinomas that demonstrated these alterations was unable to find microdeletions, insertions, or point mutations of either gene. HRPT2 gene is a putative tumor suppressor gene, which maps to chromosome 1q25-q31 and whose germline mutation is autosomally inherited in HPT-JT syndrome. HPT-JT is an unusual inherited disorder with high incidence of parathyroid carcinoma, as approximately 80% of patients have hyperparathyroidism and up to 15% of these patients develop parathyroid carcinoma. In addition, the syndrome includes benign fibro-osseous lesions of the mandible or maxilla and renal cysts or tumors. However, up to 20% of cases of apparently sporadic parathyroid carcinoma harbor germline HRPT2 mutations, representing unrecognized syndromic patients. Moreover, inactivating mutations of HRPT2 are found in nearly 70% of all parathyroid carcinomas. As a consequence, all patients with parathyroid carcinoma should have jaw and renal imaging studies, and if the HPT-JT syndrome is diagnosed, family members should undergo appropriate screening studies. Conversely, patients with parathyroid carcinomas should be tested for HRPT germline mutations, and if mutations are detected, family members should be offered genetic counseling and screening studies [11].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree