Distinguishing between Parkinson’s Disease and Parkinsonism
The syndrome of parkinsonism must be understood before understanding what is Parkinson’s disease (PD). Parkinsonism is defined as any combination of six specific, independent motoric features: tremor at rest, bradykinesia, rigidity, loss of postural reflexes, flexed posture, and the freezing phenomenon (where the feet are transiently “glued” to the ground). Not all six of these cardinal features need be present, but at least two should be before the diagnosis of parkinsonism is made, with at least one of them being tremor at rest or bradykinesia. Parkinsonism is divided into four categories (Table 66-1). PD or primary parkinsonism will be the principal focus of this chapter; not only as it is the one that is most commonly encountered by the general clinician, it is also the one on which much research has been expended and the one we know the most about. The great majority of cases of primary parkinsonism are sporadic, but in the last decade, several gene mutations have been discovered to cause PD (Table 66-2). Whether genetic or idiopathic in etiology, the common denominator is that this group of primary parkinsonism is not caused by known insults to the brain (the main feature of secondary parkinsonism) and is not associated with other motoric neurological features (the main feature of Parkinson-plus syndromes). The uncovering of genetic causes of primary parkinsonism has shed light on probable pathogenic mechanisms that may be a factor in even the more common idiopathic cases of PD. It may even turn out that many of the idiopathic cases will be linked to gene mutations, discoveries yet to be made. Although the term “idiopathic PD” has been applied to primary parkinsonism, the fact that there are known genetic causes should encourage us to adopt the term “primary parkinsonism” rather than “idiopathic parkinsonism.”
Primary parkinsonism (Parkinson’s disease) |
Sporadic |
Known genetic etiology (see Table 66-2) |
Secondary parkinsonism (environmental etiology) |
Drugs |
Dopamine receptor blockers (most commonly antipsychotic medications) |
Dopamine storage depletors (reserpine) |
Postencephalitic |
Toxins—Mn, CO, MPTP, cyanide |
Vascular |
Brain tumors |
Head trauma |
Normal pressure hydrocephalus |
Parkinsonism-plus syndromes |
Progressive supranuclear palsy (PSP) |
Multiple system atrophy (MSA) |
Cortical–basal ganglionic degeneration (CBGD) |
Diffuse Lewy body disease (DLBD) |
Parkinson–dementia–ALS complex of Guam |
Progressive pallidal atrophy |
Heredodegenerative disorders |
Alzheimer’s disease |
Wilson disease |
Huntington disease |
Frontotemporal dementia on chromosome 17 |
X-linked dystonia-parkinsonism (in Filipino men; known as lubag) |
NAME | GENE SYMBOL | PROTEIN | CHROMOSOME |
|---|---|---|---|
Autosomal Dominant Transmission | |||
PARK1/PARK4 | SNCA | α-synuclein | 4q21.3 |
PARK5 | UCH-L1 | Ubiquitin C-terminal hydrolase-L1 | 4p14 |
PARK8 | LRRK2 | Leucine rich repeat kinase 2 | 12p11.2–q13.1 |
GBA | β-glucocerebrosidase | 1q21 | |
Dopa-responsive dystonia | GTP cyclohydrolase 1 | 14q22.1–q22.2 | |
Autosomal Recessive Transmission | |||
PARK2 | PRKN | Parkin (ubiquitin ligase) | 6q25.2–q27 |
PARK6 | PINK1 | PTEN-induced kinase 1 (PINK1) | 1p35–p36 |
PARK7 | DJ-1 | DJ-1 | 1p36 |
PARK9 | ATP13A2 | ATPase | 1p32 |
Tyrosine hydroxylase deficiency | 11p11.5 |
Three of the most helpful clues that one is likely dealing with a category of parkinsonism other than PD would be (1) a symmetrical onset of symptoms (PD often begins on one side of the body), (2) a lack of a substantial clinical response to adequate levodopa therapy, and (3) the absence of rest tremor. The presence of any of these features does not necessarily exclude the diagnosis of PD, but the likelihood that the cause belongs to another category of parkinsonism is high. The clinical features suggesting a diagnosis favoring the other parkinsonian disorders and not PD are listed in Table 66-3. One common misdiagnosis is tremor owing to essential tremor, which can even be unilateral, although it more commonly is bilateral. Helpful in the diagnosis is that the tremor caused by PD is a rest tremor, whereas essential tremor is not present at rest, but appears with holding the arms in front of the body and increases in amplitude with intention activity of the arm, such as with handwriting or performing the finger-to-nose maneuver.
LIKELY DIAGNOSIS | |
|---|---|
History of: | |
Encephalitis | Postencephalitic |
Exposure to carbon monoxide, manganese, or other toxins | Toxin-induced |
Recent exposure to neuroleptic medication | Drug-induced |
Onset of parkinsonian symptoms following: | |
Head trauma | Posttraumatic |
Stroke | Vascular |
Presence on examination of: | |
Cerebellar ataxia | OPCA, MSA |
Loss of downward ocular movements | PSP |
Pronounced postural hypotension not because of concurrent medication | MSA |
Pronounced unilateral rigidity with or without dystonia, apraxia, cortical sensory loss, alien limb | CBGD |
Myoclonus | CBGD, MSA |
Falling or freezing of gait early in the course of the disease | PSP |
Autonomic dysfunction not because of medications | MSA |
Excessive drooling of saliva | MSA |
Early dementia or hallucinations from medications | DLBD |
Dystonia induced with low-dose levodopa | MSA |
Neuroimaging (MRI or CT scan) revealing: | |
Lacunar infarcts | Vascular |
Capacious cerebral ventricles | NPH |
Cerebellar atrophy | OPCA, MSA |
Atrophy of the midbrain or other parts of the brainstem | PSP, MSA |
Effect of medication: | |
Poor response to levodopa | PSP, MSA, CBGD, Vascular, NPH |
No dyskinesias despite high-dose levodopa | Same as above |
PD begins insidiously and gradually worsens. Symptoms, such as rest tremor, can be intermittent at the beginning, becoming present only in stressful situations. Patients with PD can live 20 or more years, depending on the age at onset; the mortality rate is approximately 1.5 times that of normal individuals of the same age. Death in PD is usually because of some concurrent unrelated illness or owing to the effects of decreased mobility, aspiration, or increased falling with subsequent physical injury. The Parkinson-plus syndromes typically progress at a faster rate and often cause death within 9 years. Thus, the diagnosis of PD is of prognostic importance, as well as of therapeutic significance, because it almost always responds to at least a moderate degree to levodopa therapy, whereas the Parkinson-plus disorders do not. While it may be difficult to distinguish between PD and Parkinson-plus syndromes in the early stages of the illness, with disease progression over time, the clinical distinctions of the Parkinson-plus disorders become more apparent with the development of other neurological findings, such as cerebellar ataxia, loss of downward ocular movements, and autonomic dysfunction (e.g., postural hypotension, loss of bladder control, and impotence).
There are no practical diagnostic laboratory tests for PD, and the diagnosis rests on the clinical features or by excluding some of the other causes of parkinsonism. The research tool of fluorodopa positron emission tomography measures levodopa uptake into dopamine nerve terminals, and this shows a decline of approximately 5% per year of the striatal uptake. A similar result is seen using ligands for the dopamine transporter, either by positron emission tomography or by single photon emission computed tomography; these ligands also label the dopamine nerve terminals. All these neuroimaging techniques reveal decreased dopaminergic nerve terminals in the striatum in both PD and the Parkinson-plus syndromes, and do not distinguish between them. A substantial response to levodopa is most helpful in the differential diagnosis, indicating presynaptic dopamine deficiency with intact postsynaptic dopamine receptors, features typical for PD.
The development of dementia in a patient with parkinsonism remains a difficult differential diagnosis. If the patient’s parkinsonian features did not respond to levodopa, the diagnosis is likely to be Alzheimer’s disease, which can occasionally present with parkinsonism. If the presenting parkinsonism responded to levodopa, and the patient developed dementia over time, the diagnosis could be either PD dementia (PDD) or diffuse Lewy body disease (DLBD), also known as dementia with Lewy bodies. The nosologic distinction is less of substance and more of useful categorization. The term PDD is used if the symptoms of PD have been present for at least 1 year before dementia develops. The term DLBD is used if the symptoms of PD have been present less than 1 year before onset of dementia, or if dementia presents with the onset of parkinsonism. A major feature of PDD and DLBD is the presence of hallucinations. Without hallucinations, other types of dementias should be considered, including vascular disease, Alzheimer’s disease, and frontotemporal dementia. DLBD is a condition where Lewy bodies are present in the cerebral cortex as well as in the brainstem nuclei. The heredodegenerative disease, known as frontotemporal dementia, is an autosomal dominant disorder caused by mutations of the tau gene or the progranulin gene on chromosome 17; the full syndrome presents with dementia, loss of inhibition, parkinsonism, and sometimes muscle wasting. PDD is associated with aging and increased duration of PD. The prevalence of PDD is approximately 20%, but the likelihood of developing dementia eventually in a patient with PD is much greater, with the highest estimate around 78%.
Some adults may develop a more benign form of PD, in which the symptoms respond to very low-dose levodopa, and the disease does not worsen severely with time. This form is usually caused by the autosomal dominant disorder known as dopa-responsive dystonia, which typically begins in childhood as a dystonia. But when it starts in adult life, it can present with parkinsonism. There is no neuronal degeneration. The pathogenesis is because of a biochemical deficiency involving dopamine synthesis. The gene defect is for an enzyme, GTP cyclohydrolase 1, required to synthesize the cofactor for tyrosine hydroxylase activity, the crucial rate-limiting first step in the synthesis of dopamine and norepinephrine. Infantile parkinsonism is caused by the autosomal recessive deficiency of tyrosine hydroxylase, another cause of a biochemical dopamine-deficiency disorder. Young-onset PD—less than 40 years of age, but some use a cut-off of 50 years—usually worsens more slowly than those with older onset. But these young-onset patients are more likely to develop motor complications from levodopa therapy (see below).
Pathology of Parkinson’s Disease and Parkinson-Plus Syndromes
Parkinson’s disease and the Parkinson-plus syndromes have in common a degeneration of substantia nigra pars compacta dopaminergic neurons, with a resulting deficiency of striatal dopamine caused by loss of the nigrostriatal neurons. Accompanying this neuronal loss in the nigra is an increase in glial cells and a loss of the neuromelanin in the nigra, because neuromelanin is normally contained in the dopaminergic neurons. In PD, intracytoplasmic inclusions, called Lewy bodies, are usually present in many of the surviving neurons. It is recognized today that not all patients with PD have Lewy bodies, those with a homozygous mutation in the parkin (PARK2) gene, mainly patients with young-onset PD, have nigral neuronal degeneration without Lewy bodies. Lewy bodies contain many proteins, including the fibrillar form of the protein α-synuclein. Immunostaining for α-synuclein is utilized today as the most sensitive histologic method to detect Lewy bodies. Recent research has shown that Lewy neurites (α-synuclein fibers in axons) first appear in the medulla and the olfactory bulb, and over time become present in a rostral manner up the brainstem, from medulla to pons to midbrain, and then into the thalamus and cerebral cortex. Thus, Lewy neurites (and Lewy bodies) do not start in the substantia nigra, which is located in the midbrain. There are no Lewy bodies in the Parkinson-plus syndromes.
A pathological feature of multiple system atrophy (MSA) is the presence of inclusions in oligodendroglia; these inclusions also contain α-synuclein. PSP and CBGD contain tau filaments, and these two diseases share similar clinical features, especially in the late stages of these diseases. PSP shows neurofibrillary tangles in the substantia nigra and other nuclei, while CBGD shows ballooned neurons, especially in areas of the cerebral cortex.
Cause and Pathogenesis of Parkinson’s Disease and Parkinson-Plus Syndromes
Other than known genetic causes of PD (Table 66-2), the etiology of these disorders remains unknown. Alterations in the tau gene have been implicated for PSP and CBGD. Three of the identified genes causing PD (PARK1, PARK2, PARK5) point to an impairment of protein degradation with a build-up of toxic proteins that cannot be degraded via the ubiquitin–proteasomal pathway. This has led to the concept that perhaps most, if not all, cases of sporadic PD have an impairment of protein degradation. While PARK1 represents mutations in the gene for α-synuclein, triplications and duplications of the chromosomal area for the α-synuclein gene (PARK4) also cause PD, indicating that accumulation of wild-type α-synuclein, and not just gene mutations of this protein are capable of causing neurodegeneration. A heterozygotic mutation in the gene for the lysosomal enzymes, β-glucocerebrosidase and a lysosomal ATPase (PARK9), indicates that faulty degradation of substrates can be an important pathogenic factor. One gene (PARK6) is involved with mitochondrial function, suggesting impaired energy metabolism can lead to PD. PARK7 is a gene for a protein that fights oxidative stress; thus supporting the notion that increased oxidative stress can be a risk factor for PD.
The most common gene mutation causing PD is PARK8, especially the G2019S mutation; approximately 5% of familial cases and up to 2% of nonfamilial cases have this mutation. There is ethnic disproportion of specific mutations in the LRRK2 gene. The G2019S mutation is highly prevalent in North African Arabs, Portuguese, Spanish, and Ashkenazi Jews. In the Chinese, the G2385R mutation is the most common. LRRK2 is a complex protein with several functional domains, and mutations in any of them can lead to PD. It is not clear how such mutations cause the disease.
Because accumulated α-synuclein can cause porosity in the synaptic vesicle membrane, resulting in an outward leakage of stored dopamine, the increased cytosolic dopamine can auto-oxidize to form oxyradicals, such as dopamine quinone, to cause cell damage. Other pathogenic mechanisms being considered are (1) damage to the protein degradation properties of lysosomes leading to protein accumulation and aggregation; (2) other effects from oxidative stress, such as the reaction of oxyradicals with nitric oxide to form the highly reactive peroxynitrite radical; (3) impaired mitochondria leading to both reduced ATP production and accumulation of electrons that aggravate oxidative stress, with the final outcome being apoptosis and cell death; and (4) inflammatory changes in the nigra producing cytokines that augment apoptosis. These concepts on pathogenesis are leading researchers to test agents that affect these potential mechanisms in an attempt to reduce the rate of neurodegeneration in PD.
Epidemiology and Clinical Features of Parkinson’s Disease
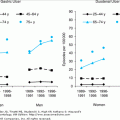
Although PD can develop at any age, it is most common in older adults, with a peak age at onset around 60 years. The likelihood of developing PD increases with age, with a lifetime risk of approximately 2%. A positive family history doubles the risk of developing PD to 4%. Twin studies indicate that PD with an onset before the age of 50 years is more likely to have a genetic relationship than for patients with an older age at onset.
The early symptoms and signs of PD are rest tremor, bradykinesia, and rigidity; these are related to progressive loss of nigrostriatal dopamine. These signs and symptoms result from dopamine deficiency and are usually correctable by levodopa and dopamine agonists. As PD progresses over time, non-dopamine-related symptoms develop, such as flexed posture, the freezing phenomenon, and loss of postural reflexes; these do not respond well to levodopa therapy. Moreover, increasing bradykinesia that is not responsive to levodopa can appear as the disease worsens. All these intractable symptoms lead to disability.