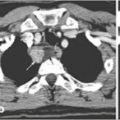
Fig. 15.1
MIBI scan (technetium and subtraction images): large inferior right parathyroid lesion that histologically proved to be parathyroid carcinoma. From [16] with permission
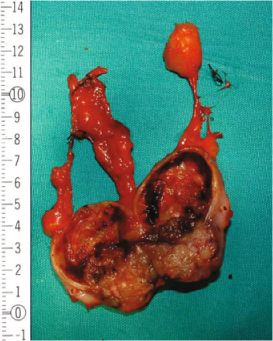
Fig. 15.2
Surgical specimen: parathyroid carcinoma; necrosis and degenerative changes are also present (the specimen has been sectioned longitudinally). From [16] with permission
The diagnosis is usually confirmed postoperatively, although in the absence of unequivocal features it may be challenging; in these cases, it may be confirmed only by the presence of distant or nodal metastases at a prolonged follow-up.
The most used histopathological criteria to diagnose parathyroid carcinoma have been established by Schantz and Castleman in 1973 [18]: presence of uniform sheets of chief cells arranged in a lobular pattern separated by dense, thick fibrous trabeculae that extend into and divide the gland; atypical mitotic figures within tumor cells and vascular or capsular invasion (Fig. 15.3).
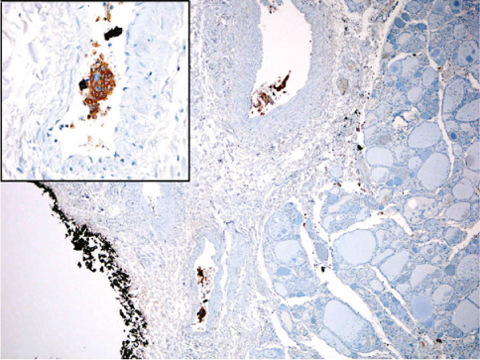

Fig. 15.3
Photomicrograph of a histological section of parathyroid carcinoma. Vascular invasion is present in the soft tissue surrounding the thyroid gland (chromogranin, ×50). Inset: particular of the invaded vessels (chromogranin, ×400). From [16] with permission
The epithelial cells of parathyroid carcinoma are larger than normal cells; mitotic activity is present in 80% of carcinomas; a high mitotic rate (>5 per 50 high-power fields) and atypical mitoses indicate an increased risk of malignant behavior; giant cells and/or focal necrosis may be present too [5, 14]. Nonetheless, degenerative changes into a non-malignant gland may closely mimic the features of a parathyroid carcinoma. However, adopting current histopathological criteria, only capsular and adjacent tissue invasion (present in 60% of cases), vascular invasion (10–15%) and perineural space invasion (rarely present) appear to correlate best with tumor recurrence and metastatic course, and can be considered pathognomonic of malignancy.
True parathyroid carcinoma should be distinguished from parathyromatosis (implantation of parathyroid tissue following intraoperative capsular rupture or incomplete excision of a parathyroid benign lesion), and “atypical parathyroid adenoma”, according to the presence of vascular and perineural space invasion.
In fact, histology alone cannot diagnose all cases, since a proportion of parathyroid tumors that do not meet the histopathological criteria for carcinoma may recur or (rarely) metastasize [6], while only a subset of tumors classified as malignant at histopathological examination demonstrate recurrent disease or distant metastases.
For these reasons, several other techniques (DNA measurement by flow cytometry, Ki67, immunohistochemical staining of Rb, p27) have been used to confirm the diagnosis. Recently the loss of parafibromin immunostaining has gained popularity because of its sensitivity (68–96%) and specificity (99%); also the adenomatous polyposis coli and the protein gene product 9.5 (PGP9.5) have shown a promising diagnostic role [8].
15.5 Medical and Surgical Treatment
Parathyroid carcinoma tends to invade the surrounding structures and to spread to regional lymph nodes (30% of cases); it may also disseminate hematogenously, with lung (40%), liver (10%) and bones metastases [4]. The prognosis is quite variable, although it usually has a slow, indolent but progressive course because of the rather low malignant potential, with a reported cumulative 5 and 10-year survival of 86% and 49%, respectively [2].
The most important factor affecting prognosis is the completeness of tumor resection; thus, the most effective treatment is surgery [4, 12]. However, since patients generally die from metabolic complications of uncontrollable hypercalcemia rather than from tumor burden, medical treatment aimed to lower hypercalcemia has a pivotal role. Furthermore, whenever possible, severe hypercalcemia should be corrected before surgery because of the risk of arrhythmias during anesthesia induction.
Hypercalcemia should be treated with urgent and aggressive restoration of fluid volume (these patients are significantly dehydrated). Loop diuretics (furosemide) are given to increase renal calcium excretion. Bisphosphonates (drugs that inhibit osteoclast-mediated bone resorption by incorporation into the bone matrix) are effective but they lose efficacy over time [4]. Pamidronate, infused in doses of 30–90 mg/day over 2–4 hours, is effective, at least transiently: responses last for 1–3 weeks and the treatment can be repeated. However, possible complications of bisphosphonates are represented by avascular necrosis of the jaw and acute renal failure.
Recently, new drugs (calcimimetics) have been developed for PTH-related hypercalcemia. Cinacalcet acts as an effective allosteric modulator of calciumsensing receptors that are responsible for the regulation of PTH secretion. It binds to the calcium receptors on the surface of parathyroid cells and increases the receptor sensitivity to extracellular calcium and subsequently reduces serum PTH and calcium levels. Cinacalcet is administrated orally (30–60 mg) and it is well tolerated; however, it does not modify the course of parathyroid cancer. Therefore, it cannot replace surgical intervention in the case of resectable disease. This drug can potentially alleviate the consequences of hypercalcemia in patients with widely metastatic disease or renal insufficiency.
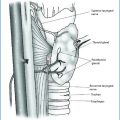
Thus, the gold standard therapy is the radical surgery, with an en bloc resection of the primary lesion at the time of the initial operation [12, 19–21], with the excision of the adjacent ipsilateral thyroid lobe in continuity with the tumor, along with minimal manipulation, in order to achieve a complete resection with disease-free margins (Fig. 15.4). Any capsular rupture of the tumor and neoplastic spillage should be avoided, since seeding leads to recurrence [1, 12]. The removal of the ipsilateral normal parathyroid has also been suggested, in order to eliminate a possible adjacent source of PTH secretion that could be a confounding factor in case of recurrence [1]. This strategy may also reduce the risk of metachronous multiglandular involvement in the case of HPT-JT-related parathyroid carcinoma, which represents one third of the cases of apparent sporadic parathyroid carcinoma [11]. The recurrent laryngeal nerve — in the absence of an unequivocal sign of infiltration — should be preserved by means of a careful dissection, whenever possible; therefore, the knowledge of the preoperative status of the vocal cord mobility is very useful. To the contrary, if the nerve is infiltrated, its sacrifice might be necessary.
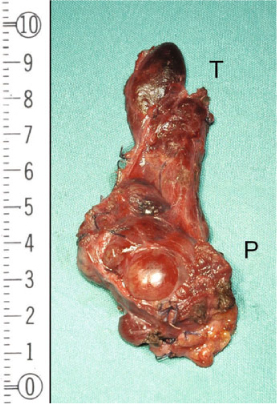

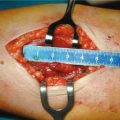
Fig. 15.4




Surgical specimen: parathyroid carcinoma — en bloc resection. The parathyroid carcinoma (P) has been removed en bloc with the omolateral thyroid lobe (T) to avoid any spillage
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree