Palliative Care: Introduction
Our society is facing one of the largest public health challenges in its history—the growth of the population of older adults. Improvements in public health, the discovery of antibiotics, and advances in modern medicine have resulted in unprecedented gains in human longevity. For most Americans, the years after the age of 65 are a time of good health, independence, and integration of one’s life’s work and experience. Eventually, however, most adults will develop one or more chronic illnesses with which they may live for many years before they die. More than three-quarters of deaths in the United States result from chronic diseases of the heart, lungs, brain, and other vital organs. Even cancer, which accounts for nearly a quarter of U.S. deaths, has become a chronic, multiyear illness for many. For a minority of patients with serious illness (e.g., metastatic colon cancer), the time following diagnosis is characterized by a stable period of relatively good functional and cognitive performance, followed by a predictable and short period of functional and clinical decline. However, for most patients with advanced illness (e.g., heart or lung disease, dementia, stroke, neuromuscular degenerative diseases, and many cancers), the time following diagnosis is characterized by months to years of physical and psychological symptom distress, progressive functional dependence and frailty, considerable family support needs, and high health care resource use. Indeed, as the population continues to age, most physicians will be caring for chronically ill individuals whose medical care is characterized by high degrees of complexity, lengthy duration of illness, and intermittent acute exacerbations interspersed with periods of relative stability. Abundant evidence suggests that the advanced stages of disease for most are characterized by inadequately treated physical distress; fragmented care systems; poor communication between physicians, patients, and families; and enormous strains on family caregiver and support systems.
The Experience of Serious Illness for Patients and Their Families
Whereas a century ago, most adults died suddenly as a result of an acute infection or accident, the leading causes of death today are chronic illness such as heart disease, cancer, stroke, and dementia. Accompanying this shift in the causes of death has been a corresponding change in the location of death. Whereas in the early part of the 20th century, most persons died at home, today, most Americans will die in an institution (57% in hospitals and 17% in nursing homes). The reasons for this shift in location of death are complex but appear to be related to health system and reimbursement structures that promote hospital-based care and provide relatively little support for needed home care and custodial care services. Whereas the majority of Americans will die in an institution, these statistics hide the fact that most of an older person’s last months and years are still spent at home in the care of family members, with hospitalization and/or nursing home placement occurring only near the very end of life. National statistics also obscure the variability in the experience of dying. For example, need for institutionalization or paid formal caregiving in the last months of life is much higher among the poor and women. Similarly, persons suffering from cognitive impairment and dementia are much more likely to spend their last days in a nursing home compared to cognitively intact elderly persons dying from nondementing illnesses.
Multiple studies have demonstrated that the clinical care of older adults with serious and advanced illness is in need of improvement. Studies have suggested that the prevalence of significant pain in community-dwelling older adults may be as high as 56% and that almost one-fifth of older adults take analgesic medications on a regular basis. Similarly, it has been suggested that 45% to 80% of nursing home residents have substantial pain and that many of these patients have multiple pain complaints and multiple potential sources of pain. Available data also point to a high prevalence of nonpain symptoms in older adults with serious and chronic illness. In retrospective interviews with family members of patients who died of noncancer illnesses in the United Kingdom, 67% of patients experienced moderate-to-severe pain, 49% had trouble breathing, 27% reported nausea, 36% reported depression, and 36% reported sleep disturbances. Similarly, SUPPORT (Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments), a study of 9 105 seriously ill hospitalized adults, found that after 1 week in the hospital, among patients who could be interviewed, about one in two patients reported pain, nearly one in six reported moderate-to-severe pain at least half of the time, and nearly one in six patients with pain were dissatisfied with its control. In subanalyses of patients with heart failure, end-stage liver disease, lung cancer, and chronic obstructive pulmonary disease (COPD), more than 20% of patients consistently experienced severe dyspnea during the 6 months prior to death. A companion study to SUPPORT reported almost identical pain findings for a cohort of hospitalized patients aged 80 years and older and also noted a high prevalence of anxiety and depression in the last 6 months of life.
The burdens of serious and chronic illness extend beyond patients to their families and friends. SUPPORT found that more than one-third of patients needed a large amount of family caregiving, which was not adequately provided for by the health care system. Similarly, another study of just less than 900 caregivers of persons with advance illness reported that more than one-third of these caregivers reported substantial stress. Furthermore, 86% of these respondents stated that they needed more help than what they were currently receiving or could afford including assistance. These needs included transportation (62%), homemaking (55%), nursing (28%), or personal care (26%). Caregiving has also been shown to be an independent risk factor for death, major depression, and associated comorbidities.
Palliative Care
Palliative care is interdisciplinary care focused on the relief of suffering and achieving the best possible quality of life for patients and their loved ones. It involves formal symptom assessment and treatment, aid with decision making and establishing goals of care, practical support for patients and their caregivers, mobilization of community support and resources to ensure secure and safe living environments, and collaborative and seamless models of care (hospital, home, nursing homes, and hospice). It is offered simultaneously with life-prolonging and curative therapies for persons living with serious, complex, and eventually terminal illness. Palliative care was recently approved as a subspecialty of internal medicine, family medicine, and seven other parent boards.
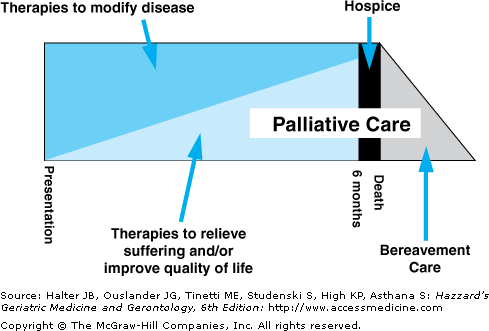
Perhaps because it is codified in the Medicare hospice benefit, palliative care, or more typically hospice, traditionally has been conceptualized as care that should be provided only at the end of life when treatments directed at the cure of disease and life prolongation are no longer effective. This artificial dichotomy (cure vs. comfort) ignores the fact that the overwhelming majority of older adults living with advanced chronic illness require both life-prolonging and palliative treatments and that the forced choice of cure versus comfort results not only in reflexive, burdensome, and costly life-prolonging treatments long after the time that these are beneficial to the patient, but also in preventable suffering during all stages of an advanced illness. For example, a frail 88-year-old woman with advanced heart failure, Parkinson’s disease, diabetes, and deconditioning after hospitalization for pneumonia typically requires life-prolonging measures (medications for heart failure, oxygen, insulin, and antibiotics), preventive measures (influenza vaccinations), rehabilitation (home physical and occupational therapy to restore bed–chair mobility), and palliative care (setting goals of medical care; treatment of anxiety and depression; pharmacologic [psychostimulants] and nonpharmacologic [energy conservation therapies] management of fatigue; diuretics, oxygen, and opioids for breathlessness; and advance care planning and appointment of a health care proxy). Thus, the model of palliative care that patients and families require through the many-year course of illness, and the one promulgated in this chapter, is care focused on the simultaneous mix of beneficial (i.e., effective) life-prolonging treatments, palliation of symptoms, rehabilitation, and support for family caregivers, with the nature of these treatments varying in response to patient needs and preferences (Figure 31-1).
Palliative care for geriatric patients differs from what is usually appropriate in younger adults because of the nature and duration of chronic illness during old age. Specifically, the many-year duration of most geriatric chronic illnesses, the high prevalence of long-term functional and cognitive impairment, and the need for long-term caregivers are less common and rarely present in younger populations. Indeed, palliative care for younger adults is typically focused on the identification of symptoms associated with either a specific advanced terminal illness and its immediate manifestations or signs of poor prognosis/imminent death. Conversely, many of the characteristics of advanced old age (frailty, functional dependence, cognitive impairment, multiple comorbidities, and symptom distress) are multifactorial in etiology and are present for many years. In the frail elderly, disease-specific treatments may ameliorate or lessen the burdens of frailty, dependence, and symptom distress but are unlikely to eliminate them. Thus, palliative care for the elderly is most appropriately centered on the identification and amelioration of functional and cognitive impairment, development of frailty and dependence on caregivers, and the associated burden of symptom distress rather than, as is typical in advanced cancer and traditional hospice settings, identification of specific and advanced terminal illness and its immediate manifestations or signs of poor prognosis or imminent death. In response to the unique needs of older adults, we believe that palliative care is as integral a part of geriatric medicine as is the focus on function.
Symptom Assessment and Treatment
Successful approaches to the assessment and management of pain, many physical symptoms, and some psychological symptoms have been well established in controlled clinical trials. Effective symptom management requires the evaluation and treatment of underlying etiologies, when possible; therapies specifically targeted at symptoms; and frequent reassessment after therapies are initiated. Table 31-1 details standard treatment approaches for pain and other symptoms.
SYMPTOM | ASSESSMENT | TREATMENT RECOMMENDATIONS |
|---|---|---|
Anorexia/cachexia | Assess whether symptom is owing to disease process or secondary to other symptoms (nausea and constipation). Determine if the patient is troubled by symptoms—often families, more than patients, are bothered by anorexia. | Pharmacologic approaches that have been shown to improve appetite and quality of life include corticosteroids, megestrol acetate, and the cannabinoids. Dexamethasone is advantageous in that it allows once-a-day dosing, has minimal mineralocorticoid properties, and may improve mood and energy. Megestrol acetate has been shown to be the most effective appetite stimulant with fewest side effects in randomized controlled trials for younger, but not older, adults. |
Anxiety | Is there is excessive worry, restlessness, agitation, insomnia, hyperventilation, or tachycardia? | Supportive counseling and cautious use of benzodiazepines because of increased risk of delirium in older adults. Selective seratonin reuptake inhibitor (SSRIs) if life expectancy is greater than 2 months. |
Constipation | Regular and routine assessment of constipation is critical, particularly for patients on opioids. Patients underreport constipation and life-threatening complications of fecal impaction, and perforation can develop quickly if regular bowel movements are not maintained. | With few exceptions, all patients on opioid therapy need an individualized bowel regimen. When an effective regimen is found, it must be continued for the duration of the opioid therapy. Most patients respond to a stool softener plus a stimulant. If dose escalation of the stimulant proves ineffective, addition of agents from alternate classes may be required. Agents available for the treatment of constipation are detailed below in order of suggested use. Additional recommendations are in Table 31-3. Stool softeners (ineffective alone and should be combined with other agents): docusate sodium and calcium docusate Stimulant laxatives: prune juice, senna, and bisacodyl Osmotic laxative: propylene glycol, milk of magnesia, and magnesium citrate Large-volume tap water enemas, high colonic enemas, or disimpaction may be needed in cases of severe constipation |
Delirium | Are the symptoms acute with episodes of inattention? Is there disorganized thinking or an altered level of consciousness? | Identify underlying/reversible causes. Ensure an appropriately calm environment with caregivers/family at bedside. Prescribe haloperidol (0.5 mg every 4 h as needed, titrated to symptoms) or an atypical antipsychotic. Benzodiazepines should be avoided as they have been shown to worsen delirium. |
Depression | Assess severity using validated instruments. The question “Are you depressed?” is a sensitive and specific question for patients with advanced illness. Reliable symptoms include feelings of helplessness, hopelessness, anhedonia, loss of self-esteem, worthlessness, persistent dysphoria, and suicidal ideation. Somatic symptoms are not reliable indicators of depression in this population of patients. | Supportive psychotherapy, cognitive approaches, behavioral techniques, and pharmacologic therapies have all been shown to be effective for treatment of depression in patients with advanced disease. Psychiatric consultation should be obtained in cases of severe depression. Patients should be asked specifically about suicidal ideation, and, if present, the reasons behind it should be carefully explored. Suicidal ideation often represents a measure of extreme distress in patients with advanced illness. Choice of pharmacologic therapy is often dictated by the patient’s estimated life expectancy. Psychostimulants (e.g., methylphenidate and dextroamphetamine) provide rapid treatment of symptoms (within days) with minimal side effects. Increased energy, decreased fatigue, and improved well-being have been reported with the use of psychostimulants. SSRIs are highly effective but may require 3–4 weeks to take effect. The side-effect profile of tricyclic antidepressants (sedation, dry mouth, constipation, and orthostasis) is a relative contraindication to their use in patients with advanced illness. |
Dyspnea | Assess severity using validated instrument. Treat reversible causes if possible. | Oxygen: Oxygen provides relief of dyspnea in circumstances of hypoxia but has also been shown to provide symptomatic relief in situations where hypoxia is not present. A cool breeze across the face (either from oxygen or a fan) has been shown to decrease breathlessness through stimulation of the V2 branch of the trigeminal cranial nerve. Opioids: Opioids have been demonstrated to significantly reduce breathlessness in randomized controlled trials without measurable reductions in respiratory rate or oxygen saturation. Effective doses are often lower than those used to treat pain, and tolerance has not been demonstrated to be a clinical problem. Anxiolytics: Anxiety and breathlessness are tightly intertwined. Anxiety may worsen breathlessness, and breathlessness may heighten anxiety. Benzodiazepines, reassurance, relaxation, distraction, and massage therapy may decrease anxiety and improve breathlessness. |
Fatigue | Assess severity and treat reversible causes if possible (e.g., anemia, electrolyte imbalances, infection, and hypoxia) | Pharmacologic therapy: Corticosteroids (e.g., dexamethasone) and psychostimulants (e.g., methylphenidate and dextroamphetamine) Nonpharmacologic therapies: energy conservation, frequent naps, occupational therapy, and physical therapy |
Nausea | Assess severity and probable cause using validated instrument. | Chemoreceptor trigger zone stimulation: drugs (opioids, digoxin, estrogen, and chemotherapy agents), biochemical disorders (hypercalcemia and uremia), and toxins (tumor-produced peptides, infection, radiotherapy, and abnormal metabolites) Treatment: Butyrophones/phenothiazines (e.g., haloperidol and prochlorperazine), prokinetic agents (e.g., metoclopramide), serotonergic antagonists (e.g., ondansetron), and atypical neuroleptics (olanzapine) Mechanical causes: gastric irritation (drugs, alcohol, iron, mucolytics, expectorants, and blood), tumors (external compression, intestinal obstruction, constipation, liver capsule stretch, upper bowel, genitourinary, and biliary stasis, peritoneal inflammation, and cardiac pain), and gastric distension (opioid-induced stasis) Treatment: Antihistamines (e.g., diphenhydramine), serotonergic antagonists (e.g., ondansetron), prokinetic agents (e.g., metoclopramide), and cytoprotective agents (e.g., ranitidine and omeprazole) Treatment of delayed gastric emptying/squashed stomach: prokinetic agents (e.g., metoclopramide, and cisapride if not contraindicated) Treatment of nonsurgical bowel obstruction: Octreotide is effective both for nausea and for abdominal pain resulting from bowel obstruction Intracranial processes: increased central nervous system pressure and anticipatory nausea. Treatment: corticosteroids and benzodiazepines Vestibular vertigo: local tumors, opioids, and motion sickness Treatment: acetylcholine antagonists (transdermal scopalamine and meclizine) |
Pain | Assess severity, location, character, precipitating, and alleviating factors using a validated instrument. | In general, pain medications should be administered on a standing or regular basis with “prn” or rescue doses available for breakthrough pain or pain not controlled by the standing regimen. Medications should be chosen based on patients’ initial pain level, and, if pain remains uncontrolled, clinicians should advance to the next level. Mild pain (1–4/10): Begin acetaminophen or a nonsteroidal anti-inflammatory agent in select patients (those with normal renal function and minimal risk of gastrointestinal toxicity). Do not exceed 3 or 2 g/d for those with hepatic dysfunction. If pain is not controlled by acetaminophen, initiate opioid therapy. Moderate pain (-5–6/10): Begin a short-acting standing opioid (morphine or oxycodone) and titrate every several days by 25%–50% of the current dose until pain relief is achieved. Once pain is well controlled, consider switching to a sustained release oral opioid or transdermal fentanyl (see Table 31-2 for recommended starting doses and conversion guidelines). Rescue doses employing immediate release opioids should be 10% of the 24-h total opioid dose and given every hour (oral) or every 30 min (parenteral) as needed. Because of their potency, hydromorphone and oxymorphone should be used with extreme caution in older adults with moderate pain and in most cases reserved for patients already on standing opioid therapy or those in severe pain. Acetaminophen can be employed for additive and opioid-sparing analgesia. Severe pain (8–10/10): Begin a standing short-acting opioid (morphine sulfate, oxycodone, and hydromorphone) and titrate rapidly until pain relief is obtained or intolerable side effects develop. Strongly consider a parenteral opioid and titrate by 50%–100% of the dose every 30–60 min until pain is controlled. Long-acting opioids (sustained release morphine/oxycodone and transdermal fentanyl) should typically be started only after pain is well controlled and steady state is achieved. Rescue doses employing immediate release opioids should be 10% of the 24-h total opioid dose and given every hour (oral) or every 30 min (parenteral) as needed. Methadone should only be used by clinicians experienced in its use. All patients on opioids should be started on a bowel regimen. Adjuvant agents (corticosteroids, anticonvulsants, tricyclic antidepressants, and bisphosphonates) can be used depending on the pain syndrome and response to opioids. |
Despite major advances in the treatment of pain, studies continue to demonstrate undertreatment in the majority of patients and care settings. In addition to unnecessary suffering, studies suggest that untreated pain is associated with adverse outcomes, including delirium, atelectasis, and pneumonia; deconditioning; and impaired functional recovery.
Pain is the most common symptom experienced by those with advanced illness, and successful management requires three key components: routine and standardized assessment, appropriate analgesic prescribing and use of nonpharmacologic modalities; and expert management of opioid-related side effects. Although somewhat rudimentary, The World Health Organization’s (WHO) analgesic ladder has traditionally been the framework most often cited for the management of malignant and nonmalignant persistent pain syndromes. Under the WHO analgesic ladder, patients rate their pain as mild (1–4 on a 10-point scale), moderate (5–6), or severe (7–10), and an analgesic is prescribed based on the patient’s pain level: acetaminophen, a nonsteroidal anti-inflammatory agent, or a Cox-II inhibitor for mild pain; an opioid combination product (e.g., oxycodone/acetaminophen, hydrocodone/acetaminophen, and codeine/acetaminophen) for moderate pain; and a strong opioid (morphine, oxycodone, hydromorphone, and fentanyl) for severe pain.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree