Overview of the Diagnostic and Management Problems Encountered When Malignant Type Calcifications Are Detected on the Mammogram
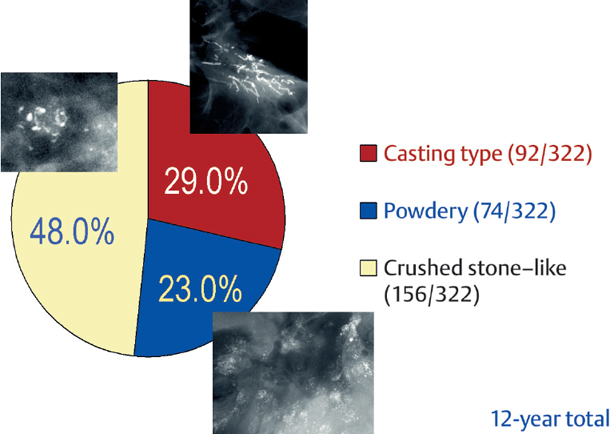
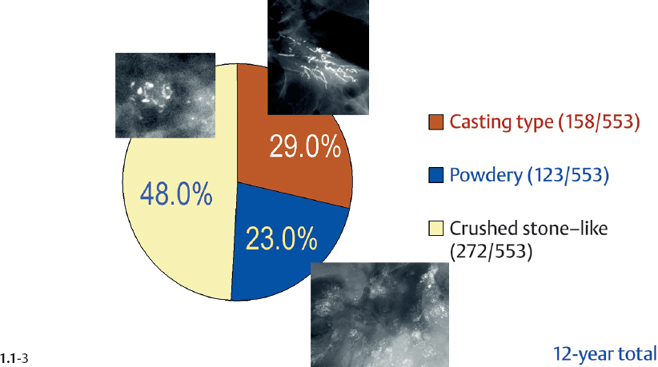
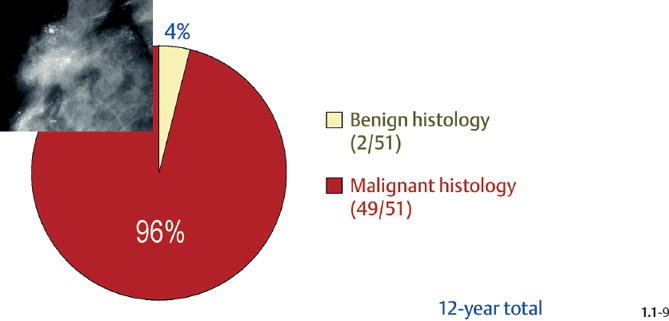
The relative frequencies of the casting type, crushed stone-like and powdery calcifications in malignant and benign breast diseases, with and without an associated tumor mass, are given in the following pie charts. The data originate from a prospective, consecutive series of patients referred to open surgical biopsy during a 12-year period at the Department of Mammography, Falun Central Hospital, Sweden.
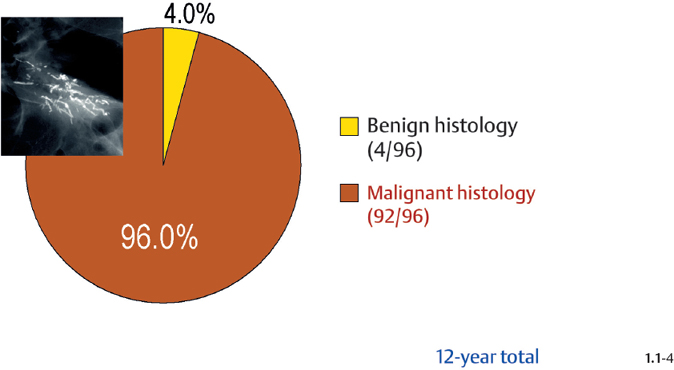
Crushed stone-like calcifications account for about half of all calcification cases referred to open surgical biopsy, regardless of whether we consider the malignant type calcifications alone or in combination with the histologically verified benign cases (Figs. 1.1-1 & 3). The relative frequency of the three types of histologically proven malignant type calcifications remains virtually unchanged regardless of whether they are associated with a tumor mass or not (Figs. 1.1-2 & 3). The value of casting type calcifications in predicting malignancy is 96% without and 99% with an associated tumor mass (Figs. 1.1-4 &5). This is not the case with crushed stone-like or powdery calcifications. As Figs. 1.1-4 to 9 demonstrate, crushed stone-like and powdery calcifications are less predictive of malignancy without an associated tumor mass. This uncertainty may cause considerable differential diagnostic problems.
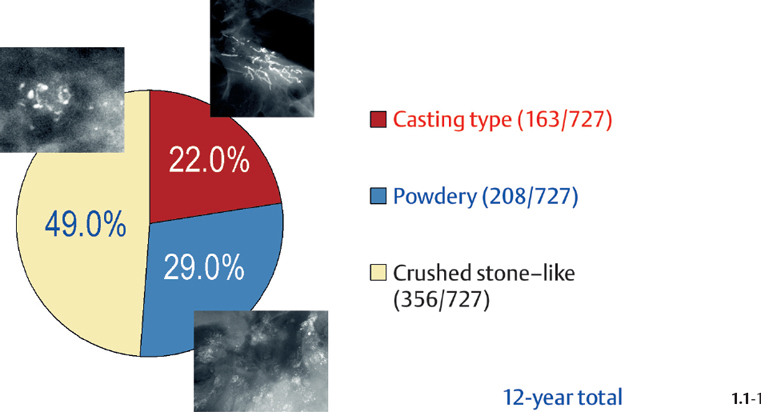
Relative Frequency of Calcification Occurrence
Distribution of All Histologically Verified Malignant Type Calcifications
Malignancy Ratio of Casting Type Calcification Cases
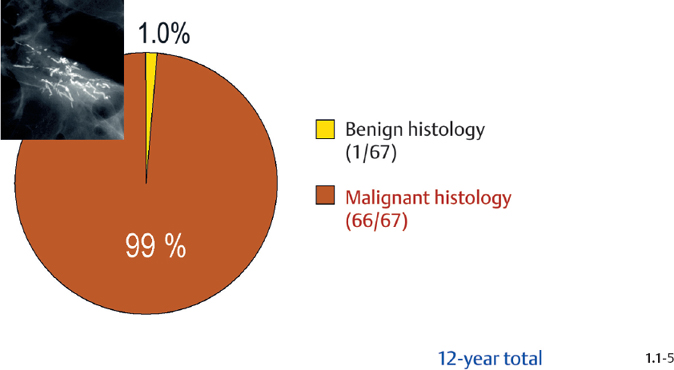
Malignancy Ratio of Crushed Stone-like / Pleomorphic Calcification Cases
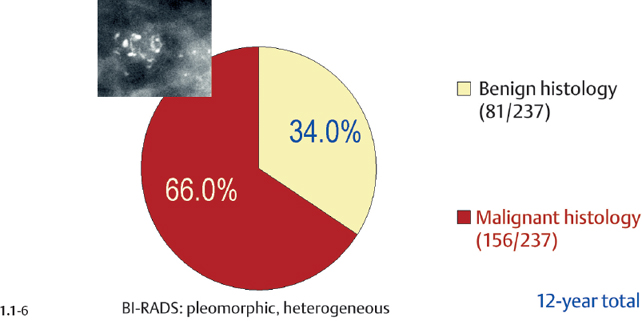
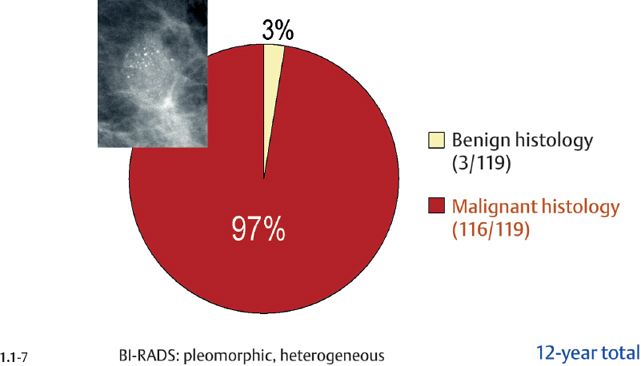
Malignancy Ratio of Powdery Calcification Cases
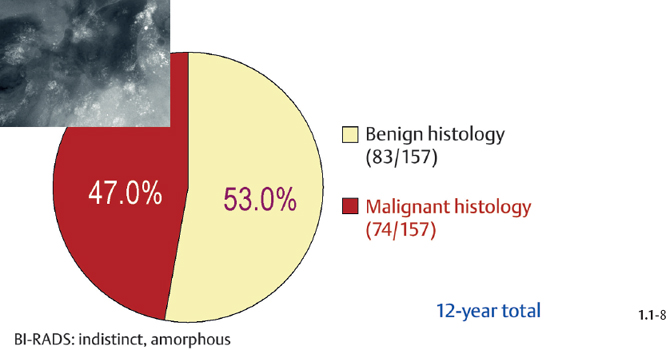
Crushed Stone-like and Powdery / Cotton Ball-like Calcifications on the Mammogram
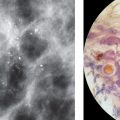
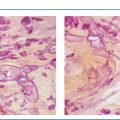
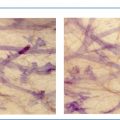
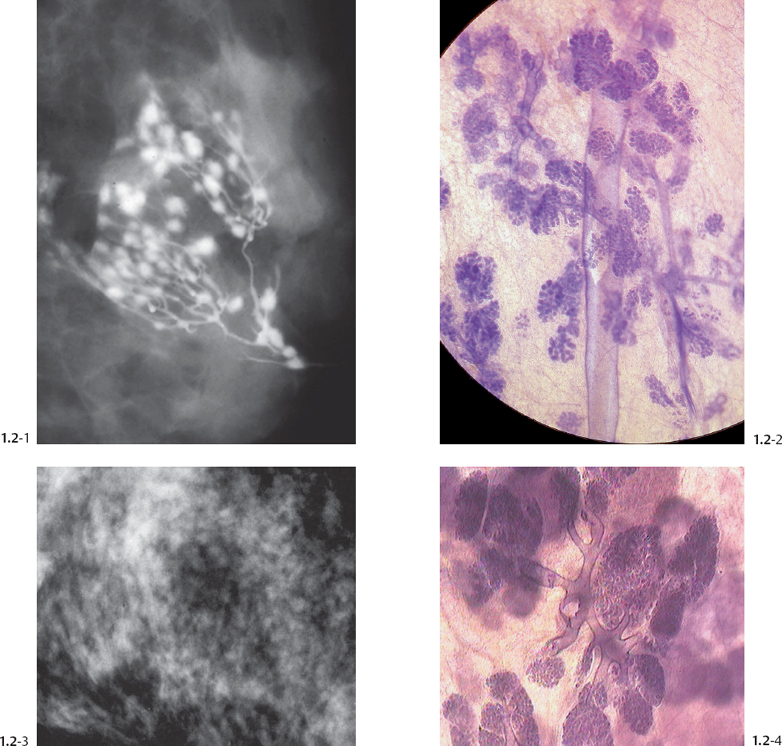
The TDLU (Figs. 1.2-1 to 4) is the origin of many heterogeneous pathological processes, including in situ carcinoma of different grades. However, most abnormalities arising within the TDLUs are benign hyperplastic breast changes. These diseases may produce proliferating cells and/or an excessive amount of fluid, either of which will distend and distort the TDLUs by increasing the intraluminal pressure. In either case calcifications may be produced within and confined to preexisting TDLUs and may be detectable on the mammogram as single or multiple clusters. These resulting calcifications can be classified into two different categories:
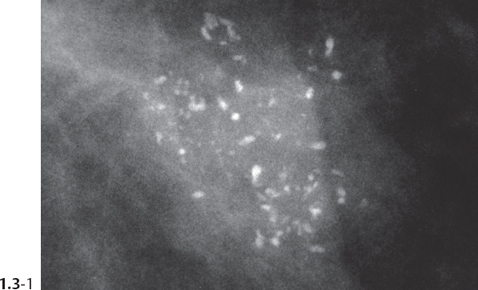
The individual calcifications are discernible, resembling crushed stones (Figs. 1.3-1 & 2); a thorough analysis of their distribution, shape, size and density will assist in determining the underlying benign or malignant pathophysiological process.
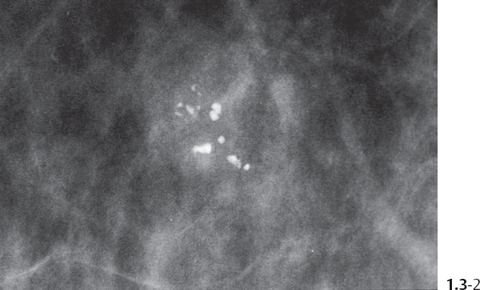
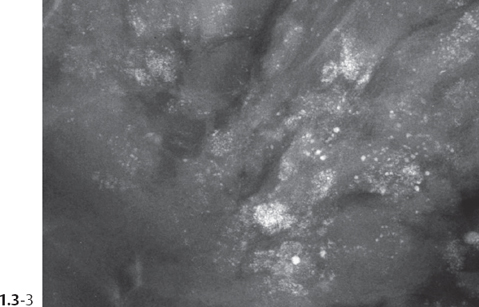
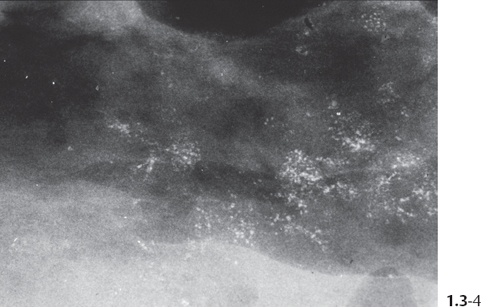
The individual calcifications are indiscernible, powdery, dusty, resembling cotton balls. The limited resolution of mammography does not reveal the individual calcification particles in powdery calcifications (Figs. 1.3-3 & 4).
Crushed stone-like and powdery calcifications seen at mammography can each represent both benign and malignant breast diseases. For both types of calcifications, benign and malignant processes mayproduce an identical appearance on the mammogram (Figs. 1.3-1 to 4).
Comment
The use of large-format subgross/thick-section histology technique is an instructive way to visualize how the morphology of the TDLU is altered by the gradual accumulation of fluid or cell proliferation/necrosis/calcifications. Comparison of subgross, 3D histological, and mammographic images is a most effective way to gain an understanding of the underlying breast diseases and their mammographic presentation. Examination of the sub-gross, thick-section 3D histological images presented in this book will demonstrate many cases in which the malignant cells are confined to the terminal ductal lobular unit (TDLU) with no ductal involvement. Use of the term “ductal” when describing these cases, although widely practiced, provides a misleading description of the location of the disease. For these cases we have used the terminology of Grade 1 or 2 or 3 in situ carcinoma. The grading system applied considers nuclear grade and presence or absence of central necrosis.1,2 The term in situ carcinoma used in this book should be considered identical to DCIS of the same grade according to the currently used terminology.
In situ carcinoma, Grade 1: low nuclear grade without central necrosis.
In situ carcinoma, Grade 2: either malignant cells with low nuclear grade and central necrosis or malignant cells with intermediate nuclear grade, with or without central necrosis.
In situ carcinoma, Grade 3: malignant cells with high nuclear grade with or without central necrosis. Cases with the cellular features of LCIS are not graded.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree