Figure 6.1 A, Normal tympanic membrane. B, tympanic membrane with mild bulging. C, tympanic membrane with moderate bulging. D, tympanic membrane with severe bulging. (Courtesy of Alejandro Hoberman.)
Clinical symptoms and signs do not differentiate specific otopathogens. The one clinical finding that is consistently associated with a specific pathogen is conjunctivitis with NTHi.
Microbiology of AOM in the era of universal immunization with pneumococcal conjugate vaccine
The pathogenesis of AOM reflects that nasopharyngeal otopathogens ascend the Eustachian tube into the middle ear. Therefore, although the etiology of individual episodes can only be established by sampling the middle ear (tympanocentesis), the spectrum of otopathogens colonizing the nasopharynx will define the microbiology of AOM.
The introduction of PCV7 reduced invasive pneumococcal disease due to the seven vaccine serotypes (4, 6B, 9V, 14, 18C, 19F, and 23F), decreased episodes of vaccine serotypes pneumococcal otitis media as well as overall clinical episodes and tympanostomy tube insertion, and impacted substantially on pneumococcal serotype distribution in the nasopharynx. In clinical trials of PCV7 (FinOM) a 34% overall reduction in pneumococcal otitis was reported; the reduction in vaccine serotype AOM was greater but an increase in episodes due to nonvaccine serotypes of S. pneumoniae was observed, providing initial evidence that “replacement” disease (that due to nonvaccine serotypes or alternative otopathogens) was significant. Initially replacement of disease due to vaccine serotype with nonvaccine serotype reduced episodes of AOM due to penicillin-nonsusceptible pneumococci. This was a result of the clustering of resistance among a limited number of pneumococcal serotypes, primarily the vaccine serotypes.
Subsequently nonvaccine serotypes, dominated by serotypes 19A and 6A, emerged. Specifically, serotype 19A became the most commonly recovered pneumococcal serotype in studies of nasopharyngeal colonization and in invasive pneumococcal disease and was a frequent cause of treatment failure in children with acute AOM. In 2010 a 13-valent pneumococcal conjugate vaccine was introduced (serotypes 1, 3, 5, 6A, 7F, and 19A), in part due to the burden of pneumococcal disease due to multidrug-resistant 19A. A decline in both nasopharyngeal colonization and invasive pneumococcal disease with serotype 19A has been observed; however, specific data on AOM is limited. Dagan has reported a decline in AOM due to the PCV13 unique serotypes in observational studies of children undergoing tympanocentesis as part of clinical management in Israel.
Knowledge of the current distribution of pathogens in children with AOM and the prevalence of penicillin-nonsusceptible S. pneumoniae or β-lactamase production among NTHi is limited. Studies of nasopharyngeal isolates in the postPCV13 ear demonstrate high prevalence of penicillin-nonsusceptible isolates of S. pneumoniae and β-lactamase production among isolates of NTHi; however, strains highly resistant to penicillin and/or ceftriaxone appear to be decreasing. Reports of isolates of NTHi with altered penicillin-binding proteins and increased minimal inhibitory concentrations for amoxicillin–clavulanate have appeared; however, such strains remain uncommon in the United States.
Treatment
Pain is a common symptom in AOM. Antibiotic therapy, even when effective, does not appear to provide symptomatic relief over the first 24 hours. Analgesics are effective for pain relief and should be prescribed regardless of whether initial management included antibiotics. Ibuprofen or acetaminophen is effective, and topical agents such as Auralgan may offer symptomatic relief. For children with severe pain, myringotomy is an effective method to attain relief. The treatment of otalgia is reviewed in Table 6.1.
| Modality | Comments |
|---|---|
| Acetaminophen, ibuprofen |
|
| Home remedies: (no controlled studies that directly address effectiveness) Distraction External application of heat or cold Oil |
|
| Topical agents: Benzocaline (Auralgan®, Americaine Otic®) Naturopathic agents (Otikon Otic Solution®) |
|
| Homeopathic agents |
|
| Narcotic analgesia with codeine or analogs |
|
| Tympanostomy/myringotomy (EBOM 227–240) |
|
The role of antibiotics in the treatment of AOM continues to undergo re-evalution. Multiple questions must be addressed to formulate a strategy for treatment of AOM.
Do children treated with antimicrobial therapy improve more quickly than those assigned to analgesia alone?
Antimicrobial therapy for AOM has reduced suppurative complications dramatically, specifically mastoiditis, over the past five decades. Similarly, in special populations such as Native Americans and Eskimo children, the prevalence of CSOM has declined in association with both the introduction of antimicrobial therapy and the improvement in public health and socioeconomic conditions. Today, AOM resolves in the majority of children without complications with or without antimicrobial therapy.
The observation that 20% to 30% of episodes are culture negative and that a proportion of children with acute bacterial otitis spontaneously clear the pathogen (approximately 15% of those with pneumococcal disease, 40% of those due to NTHi, and up to 75% of those with AOM due to M. catarrhalis) led some experts to suggest that symptomatic therapy should be the initial approach. Historically, Engelhard and associates reported greater than 70% failure in children with AOM who received myringotomy alone and Kaleida and colleagues observed a 2-fold higher failure rate among children with temperature greater than 103°F treated with myringotomy plus placebo compared with antibiotics (23.5% vs. 11.5%). They also observed an approximately 2-fold greater failure rate in children with nonsevere episodes who were treated with placebo compared with those who received amoxicillin (7.7% vs. 3.9%). However, studies by Little and coworkers challenged the impact of antimicrobial therapy on AOM symptoms. Their studies compared the outcome of AOM in children initially treated with amoxicillin with those given a prescription to be filled only if symptoms persisted for 72 hours. The authors concluded that immediate antibiotic prescription provided symptomatic benefit mainly after the first 24 hours, when symptoms were already resolving. For children who are not very unwell systemically, a wait and see approach was feasible and acceptable to parents and should substantially reduce the use of antibiotics for acute otitis media. However, both the enrollment criteria and the accuracy of diagnosis in their study were criticized. McCormick and colleagues evaluated “watchful waiting” as a strategy for children with nonsevere AOM. Increased treatment failures and persistent symptoms were observed, especially in those younger than 2 years old assigned to delayed antibiotic treatment. Although delayed resolution was observed in the cohort assigned to watchful waiting, parent satisfaction was not different among the early treatment and the initial observation groups. Increased rates of mild adverse events as well as increases in the prevalence of nonsusceptible S. pneumoniae in the nasopharynx were observed in the early treatment group.
Two recent randomized trials of initial antimicrobial therapy vs. placebo for infants and toddlers concluded that treatment reduced the time to resolution of symptoms and the overall symptom burden. Treatment failure with “rescue” antibiotic treatment and signs of persistent acute infection on otoscopic examination were more prevalent in the placebo group (Table 6.2). Table 6.3 summarizes the potential benefits and harms of initial antimicrobial therapy for AOM and provides the current recommendation from the American Academy of Pediatrics.
| Outcome | Amoxicillin–clavulanate | Placebo (n = 158) | Difference (95% CI) |
|---|---|---|---|
| Treatment failure | 30 (18.6%) | 71 (44.9%) | −26.3 (−36.5 to −16.1) |
| No improvement by day 3 | 12 (7.5%) | 22 (13.9%) | −6.5 (−13.2 to 0.3) |
| Worsening of condition | 15 (9.3%) | 32 (20.3%) | −10.9 (−18.7 to −3.2) |
| Tympanic membrane perforation | 1 (0.6%) | 5 (3.2%) | −2.5 (5.5 to 0.4) |
| “Rescue” treatment | 11 (6.8%) | 53 (33.5%) | −26.7 (−35.5 to −17.9) |
| Use of antipyretics/analgesic | 133 (84.2%) | 134 (85.9%) | −1.7 (−9.6 to 6.2)% |
| Condition | Potential for benefit | Potential for harm | Denouement |
|---|---|---|---|
| Severe symptoms | Increased likelihood of more rapid resolution of symptoms. Increased likelihood of resolution of AOM | Adverse events attributable to antibiotics, such as diarrhea, diaper dermatitis, and allergic reactions. Overuse of antibiotics leads to increased bacterial resistance. Cost of antibiotics | Preponderance of benefit over harm |
| Nonsevere bilateral AOM in young children | Increased likelihood of more rapid resolution of symptoms. Increased likelihood of resolution of AOM | Adverse events attributable to antibiotics, such as diarrhea, diaper dermatitis, and allergic reactions. Overuse of antibiotics leads to increased bacterial resistance. Cost of antibiotics | Preponderance of benefit over harm |
| Nonsevere unilateral AOM in young children | Moderately increased likelihood of more rapid resolution of symptoms with initial antibiotics. Moderately increased likelihood of resolution of AOM with initial antibiotics | Adverse events attributable to antibiotics, such as diarrhea, diaper dermatitis, and allergic reactions. Overuse of antibiotics leads to increased bacterial resistance. Cost of antibiotics | Observation becomes an alternative as the benefits and harms approach balance |
| Nonsevere AOM in older children | Slightly increased likelihood of more rapid resolution of symptoms; slightly increased likelihood of resolution of AOM | Adverse events attributable to antibiotics, such as diarrhea, rashes, and allergic reactions. Overuse of antibiotics leads to increased bacterial resistance. | Observation is an option as the benefits and harms approach balance |
Does persistence of bacterial infection within the middle ear correlate with persistence of clinical signs or symptoms?
The outcome measure selected is critical for determining the impact of antimicrobial treatment on the course of AOM. If outcome parameters such as resolution of signs and symptoms by day 7 to 10 or persistence of middle ear fluid at day 14 or 28 are selected, no differences between antimicrobial treatment and watchful waiting strategies can be consistently established. Effective antimicrobial therapy sterilizes the middle ear, resulting in a more rapid resolution of clinical signs (bulging and erythema) and symptoms (fever, earache, irritability). Therefore, evaluating outcomes within the first 3 to 5 days is necessary to demonstrate improved outcomes in antibiotic-treated cohorts as well as between antibiotic regimens.
Both Dagan and Carlin observed greater improvement in signs and symptoms in children with bacterial AOM when the middle ear fluid was sterilized by days 4–6 compared to children who had persistent middle ear infection. Figure 6.2 details the changes in clinical symptom score in children with effective antimicrobial therapy and sterilization of the middle ear compared to those with ineffective antimicrobial therapy and persistence of middle ear infection. The results also demonstrate that many children with persistent middle ear bacterial infection have decreased symptoms at days 4–6 compared to initial presentation.
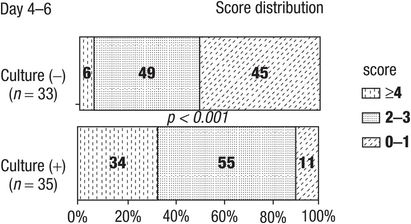
Figure 6.2 Symptoms score in children with AOM.
Is the risk of recurrence greater in children who are not initially treated with antimicrobial therapy?
Patients with clinical improvement or cure on days 4–6 but culture-positive middle ear fluid were shown to have an increased rate of recurrent AOM compared to those with culture-negative middle ear fluid and clinical improvement or cure (Figure 6.3). Molecular analysis of the otopathogens isolated at recurrence and those identified on days 4–6 found concordance in 66% of patients. These observations emphasize the benefit of bacteriologic eradication in AOM.
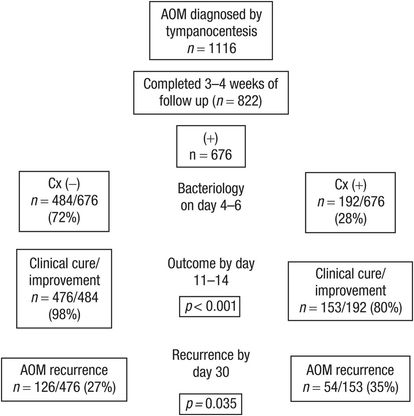
Figure 6.3 Association between eradication of middle ear pathogens during Rx and improvement in clinical symptoms. Cx = culture.
Does amoxicillin remain the initial drug of choice when the decision to use antimicrobial therapy has been made?
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





