12 Other Intraoperative Aids
Increasing evidence of the importance of accurate, reliable, and safe surgical resection of intracranial tumors has placed greater responsibility on the neuro-oncological surgeon. A wide variety of intraoperative technological advances have facilitated achievement of successful surgical resection, including co-registered image-guidance systems, intraoperative computed tomography (CT) and magnetic resonance imaging (MRI), as well as a number of other surgical adjuvant tools including intraoperative ultrasound and numerous optical imaging techniques. These other intraoperative technologies are the focus of this chapter. Some, such as intraoperative ultrasound, have a record of utilization over decades; others, such as confocal microscopy and optical coherence imaging, are relatively new to the operating room. All the technologies discussed here have been brought to clinical application, and their development is ongoing.
 Intraoperative Ultrasound
Intraoperative Ultrasound
Incorporation of ultrasound imaging into the operating room was begun more than three decades ago.1,2 The ability of ultrasound to detect subcortical and deep objects of interest such as tumors, coupled with its ease of use, ready availability, and relatively low cost, led a number of practitioners to investigate its intraoperative utility and to report encouraging preliminary experiences.1–3 The image quality in these early applications was low in comparison to today’s technology, but guidance to tumors, the ventricular system, hematomas, and foreign objects was readily demonstrated. Perhaps overshadowed by the rapid adoption of image-guidance systems co-registering more intuitively appealing and higher quality preoperative CT and MRI data sets, wider utilization of intra-operative ultrasound was limited.
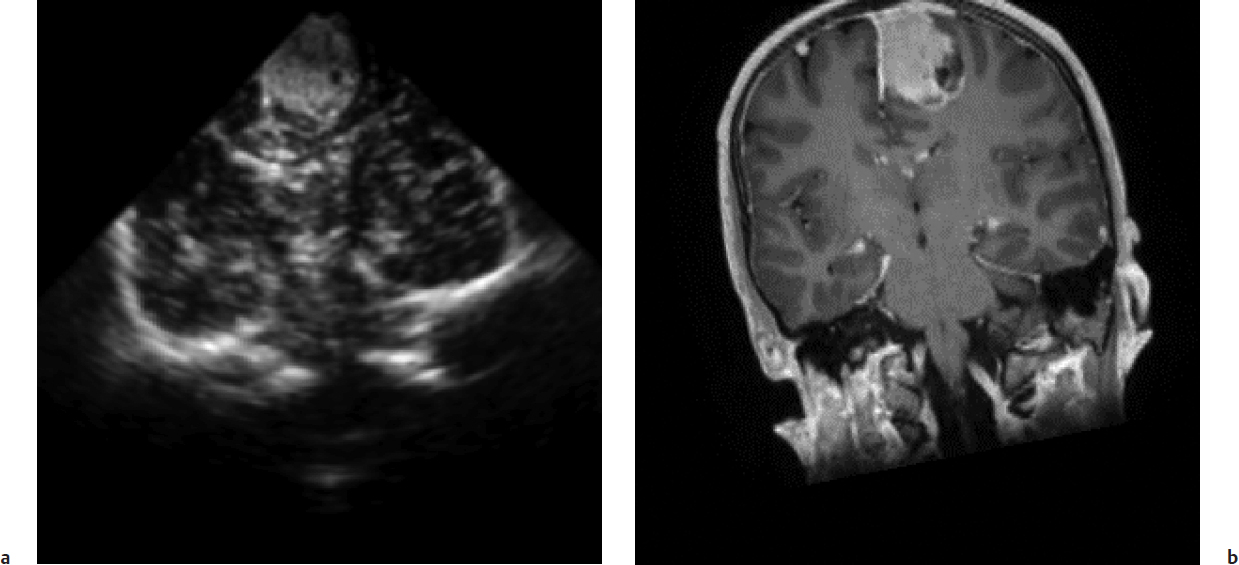
Integration of ultrasound into navigation systems that could show instrument location with respect to not just preoperative CT or MRI but also current ultrasound was soon forthcoming.4 Using the digitizer technology of neuronavigation systems, the ability was acquired to track the location and orientation of the ultrasound transducer, and in turn, through a calibration step, that of the ultrasound image with respect to the surgical field and any other co-registered images (Fig. 12.1). Intraoperative two-dimensional (2D) ultrasound image, almost always double-oblique with respect to traditional imaging triplanar display, may be difficult for the surgeon to orient, but such co-registration enables simultaneous display of the more easily interpreted corresponding CT or MRI plane.
Two immediate advantages of such intraoperative imaging are the ability to determine the extent of ongoing tumor resection and the ability to demonstrate the amount of current intraoperative registration error resulting from brain deformation or shift. Systems have been promoted for utilizing the latter capability, although they have served more to point out registration error than to actually correct it. Corrective deformation of preoperative MRI using intraoperative ultrasound information has been pursued, however, and is in development.5 As ultrasound itself improves sufficiently in image quality, navigating by the ultrasound image alone is becoming possible.
Further enabling such navigation are developments addressing the limitations of nonstandard, oblique image planes. Three-dimensional (3D) image data sets have been generated through acquisition of multiple 2D images that can then be registered to a common coordinate system and reformatted in axial, coronal, and sagittal planes. The challenges of such a strategy, including ensuring sufficient density of image data as well as the inefficiencies of acquiring such data sets, are not trivial, but they have been overcome by several groups. More recently, 3D ultrasound probes capable of acquiring full data sets more completely and efficiently have been utilized for such purposes.6 A recent comparison of clinical experience with both a 3D ultrasound system integrated into a neuro-navigation system (IGSonic, VectorVision; BrainLAB, Munich, Germany) and a nonintegrated 3D ultrasound system found that the latter was more time-efficient, but the spatial orientation enabled by navigation using reformatted images was superior.7
Progressive improvement in ultrasound image quality as well as integration into guidance systems has led to practical and effective clinical applications, and contemporary reports continue to raise the promise of inexpensive, accurate, updated intraoperative imaging. Use of an intraoperative 3D ultrasound integrated system (SonoWand, Trondheim, Norway) has been reported in over 900 operations for intraparenchymal tumor as well as cavernoma, skull base tumor, and arteriovenous malformation. Advantages include portability among operating rooms, utilization of regular surgical instruments, little additional time required, and low cost.8
Special Consideration
• Progressive improvement in ultrasound image quality as well as integration into guidance systems has led to practical and effective clinical applications.
 5-Aminolevulinic Acid–Induced Fluorescence
5-Aminolevulinic Acid–Induced Fluorescence
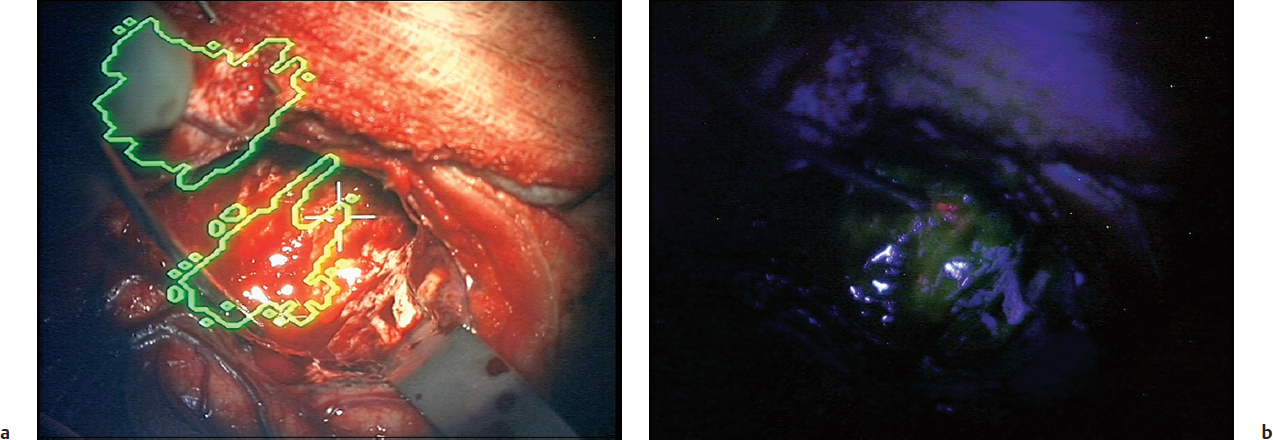
The preferential accumulation of the fluorophore protoporphyrin IX (PpIX) in tumor tissue following exogenous administration of the precursor 5-aminolevulinic acid (5-ALA) has generated excitement as a powerful intraoperative aid during glioma resection. An operating microscope adapted to enable illumination of the operative field with excitatory violet-blue light, and to optimize using filters for the perception of any resulting fluorescence, provides an intuitive, easy to use, and efficient platform for the elicitation of pink-red fluorescence in otherwise visibly indistinguishable tumor (Fig. 12.2). This was demonstrated in a C6 glioma rat brain tumor model9 and then investigated in a single-institution clinical trial of 52 patients operated on for glioblastoma. Resection of fluorescent tissue when anatomic location safely allowed it resulted in complete resection of contrast-enhancing tumor on postoperative MRI in 63% of patients, with residual (unresectable) solid fluorescent tissue intraoperatively being a negative predictive factor for survival.10
Special Consideration
• There is high correlation between contrast-enhancement on MRI and 5-ALA–induced fluorescence. Fluorescence is also correlated with higher tumor grade, cellular proliferation, metabolic imaging, and capillary density.
This group then went on to carry out a landmark, multi-institutional prospective clinical trial randomizing patients with malignant gliomas to either 5-ALA–assisted tumor resection or conventional white-light microsurgery. Primary endpoints were the number of patients with residual contrast-enhancing tumor on postoperative MRI and 6-month progression-free survival. The study was terminated at interim analysis of 270 patients, 139 of whom received 5-ALA (20 mg/kg) orally 3 hours prior to surgery and 131 of whom were operated on under white light conditions only. Complete resection of MRI contrast-enhancing tumor was achieved in 90 patients (65%) in the 5-ALA group and in 47 patients (36%) in the white light group. Six-month progression-free survival was 41% and 21.1%, respectively. Severe adverse events and adverse events within 7 days of surgery did not differ between groups.11
Special Consideration
• Fluorescence-guided resection of glioblastoma multiforme by using 5-ALA–induced porphyrins has been shown to provide higher levels of gross total resection.
A subsequent supplemental analysis of this series focused on the potential trade-off of increased risks but potential later gains associated with more extensive resection. In the prospective, randomized trial, more frequent deterioration in the National Institutes of Health Stroke Scale (NIH-SS) score was seen in the 5-ALA cohort of patients, but there was no difference in Karnofsky Performance Scale scores. Those patients with neurologic deficits that had not been responsive to steroids were most at risk of postoperative decline. Over time, differences in NIH-SS scores between the two groups became nonsignificant, and the cumulative incidence of repeat surgery was significantly less in the 5-ALA group.12
Whether 5-ALA–induced fluorescence could help in the detection of anaplastic foci in diffusely infiltrating gliomas without significant contrast-enhancement on MRI was investigated in a series of 17 patients. Eight of nine patients whose histology was that of World Health Organization (WHO) grade III tumor had focal areas of fluorescence, whereas all eight patients with WHO grade II tumors did not. Focal areas of fluorescence were found to be topographically correlated with 11C-methionine positron emission tomography (PET), and MIB-1 labeling index was significantly higher in fluorescing tissue.13
Pitfall
• Visual PpIX fluorescence tells the surgeon only about the exposed surface of the tumor; a tumor that is more than 0.25 mm below the surface or covered in blood will not be detected.
Special Consideration
• Besides high-grade gliomas, relatively few low-grade gliomas, about 80% of meningiomas, and some metastatic tumors show visible 5-ALA fluorescence.
Increasing utilization of this PpIX fluorescence–guided technology has been associated with continued improvement in rates of complete resection of tumor. A recent report described no residual contrast-enhancing tumor > 0.175 cc in 51 of 53 patients (96%) and no residual enhancement in 89%,14 results that certainly raise the bar. Another recent report focused on 52 patients with no residual contrast-enhancement on postoperative MRI and confirmed the favorable prognosis associated with no residual fluorescence at the close of surgery. Median survival in patients without residual fluorescence was 27 months, and that in patients with residual fluorescence was 17.5 months.15
All of the above series as well as most of the currently published data use subjectively assessed, visible fluorescence as visualized through one of two commercially available adapted microscopes. Quantitative measurement of PpIX levels in intracranial tumors has also been investigated. Intrinsic optical properties of tissue, including absorption and scattering, have major effects on visualization of fluorescence, and algorithms utilizing spectroscopic information can improve assessment of intraoperative tissue. One series using such technology and a fiberoptic probe that could interrogate a focal region of the surgical field over several seconds demonstrated substantially improved diagnostic performance. In a group of 14 patients with diagnoses of low- or high-grade glioma, meningioma, or metastasis, significant differences in PpIX concentration were found in all tumor types compared with normal brain. Receiver operating characteristic (ROC) curve analysis showed a classification efficiency of 87% (area under the curve [AUC] = 0.95, specificity = 92%, sensitivity = 84%) using a quantitative probe versus a classification efficiency of 66% using conventional, qualitative fluorescence imaging (AUC = 0.73, specificity = 100%, sensitivity = 47%); 81% of quantitative measurements of tissue whose fluorescence was below the surgeon’s visual perception were correctly classified in this series.16
• The increased extent of resection achievable by using fluorescence-guided resection of glioblastoma appears to be another source of non–class I evidence that the extent of resection correlates with improved survival time for glioblastoma.
More recently, more sensitive quantitative methodologies have been developed that employ wide-field imaging through the operating microscope, which is a more user-friendly and more efficient technology than the above probe. Such a methodology in which spectrally resolved data can be converted into images of absolute fluorophore concentration pixel by pixel across the surgical field has been shown to be linear, accurate, and precise relative to true values.17 The ability to do this with multiple fluorophores simultaneously has intriguing potential as new fluorophores further develop.
 Fluorescein Fluorescence
Fluorescein Fluorescence
Although the last decade has seen great interest in fluorescence guidance for cranial tumor resection, particularly using ALA-induced PpIX fluorescence, the use of fluorescence for neurosurgical guidance dates back to the work in 1948 using a Wood’s lamp for visualization of fluorescein in multiple tumor types including gliomas, meningiomas, and metastatic carcinomas.18 Fluorescein is a visible fluorophore with a main excitation peak at approximately 490 nanometers (nm; visible range approximately 400 to 550 nm) and a main emission peak at approximately 520 nm (visible range approximately 480 to 650 nm) (Fig. 12.3). Patients usually receive a dose of 200 to 1,000 g of intravenous (IV) fluorescein. More recently, various groups have utilized surgical microscopes modified for fluorescein imaging. One group visualized fluorescein staining of metastatic tumors under white light,19 and another modified a Zeiss surgical microscope by adding a blue excitation filter and a long pass filter to enable visualization of green fluorescence from fluorescein in 10 patients with high-grade gliomas.20 Various groups are using a commercial system (Zeiss OPMI, yellow 560 module) for excitation and visualization of green fluorescein fluorescence for resection of gliomas and metastases. These systems use a combination of excitation and long-pass emission filters, analogous to current clinical systems used for ALA-induced PpIX fluorescence, but optimized in the excitation and emission ranges of fluorescein.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree