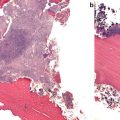
Fig. 1.1
Electron micrograph of damaged trabecula . A microcrack is present. Fibrillar structures can be observed spanning the crack as fibrils have been pulled apart from each other.
From Thurner P., et al. Engineering Fracture Mechanics 2007 [3]. Reprinted with permission from Elsevier
In cortical bone , fibers are arranged longitudinally, and new bone is formed around Haversian canals, which contain the nutrient blood supply and nerve; concentric layers of bone are laid down, yielding cylinders of bone, known as osteons, which are consolidated together by cement lines. These canals are observed in histomorphometric examinations and on images obtained from microCT as cortical pores. Their size and number tend to increase with age [4]. The basic structure of trabecular bone , at younger ages, resembles a robust scaffolding, with rods and plates of bone interconnected, around a central repository of marrow tissue. With age, trabeculae tend to thin; a reduction in number and size appears to differ by sex [4]. The combination of the honeycomb trabecular scaffolding with the surrounding dense cortical envelope allows resistance to bending forces, conferred by the cortical bone, and also to compressive forces, contributed by the trabecular bone, while maintaining lightness and so facilitating locomo tion.
Natural History of Bone Structure across the Lifecourse
Bone Mass
Bone mineral serves to strengthen the extracellular matrix, increasing bone stiffness and resistance to compression. BMD is measured as the grams of mineral per unit area or volume. Bone mass, a composite of bone mineral content (BMC) and bone size, increases throughout childhood and adolescence to peak in young adulthood (around the end of the second/start of the third decade of life), before a subsequent decline in later life due to bone loss. This bone loss accelerates further after the female menopause. Risk of osteoporosis is therefore determined by both the peak bone mass (PBM) achieved and the rate of bone loss. Attainment of a higher PBM is influenced by nutrition, physical activity, and mechanical loading during growth in addition to genetic variability, and mathematical modeling has suggested that a 10 % higher PBM will delay the onset of osteoporosis by 13 years [5].
Bone Size and Geometry
Macroscopically, the geometric properties of a bone are important. In addition to strength, the skeleton needs to be light to enable locomotion. The bending strength of a tubular structure is determined by the fourth power of its radius, and, as such, the periosteal circumference of a long bone has been demonstrated to predict up to 55 % of the variation in strength [6]. The balance between strength and weight of a bone is achieved through remodeling to increase the periosteal circumference while simultaneously increasing the area of the marrow cavity, resulting in a smaller cortical thickness for the same material weight. A thicker cortex also confers greater strength, although to a lesser extent than total cross-sectional area (CSA) of a bone . Males typically have a larger bone CSA than females, which partly results from the later pubertal growth spurt and hence completion of linear growth in boys [7]. This therefore confers greater bone strength in males compared to females. A reduction in cortical thickness occurs following the menopause, whereas a similar phenomenon is not observed in males [4], which further contributes to the failure load discrepancy between males and females in later life. Furthermore, the macroscopic shape of a bone is also adapted to the direction of normal physiological forces which it experiences. As such, the direction at which an impact force is applied to a bone will determine the likelihood of fracture; for example, a fall sideways rather than forwards is more likely to lead to a hip fracture as the direction of trauma is different to that experience from normal weight-bearing forces.
Bone Microarchitecture
Bone microarchitecture describes the structure of the cortical and trabecular compartments and can be assessed invasively by histomorphometric examination of bone biopsies or noninvasively using high-resolution peripheral quantitative computed tomography (HR-pQCT ) . Within the trabecular compartment, greater trabecular number confers higher resistance to compressive forces. Trabecular thickness is also an important determinant of deformation. While both trabecular number and trabecular thickness are important determinants of fracture risk, bone loss through trabecular number confers greater structural compromise than bone loss through trabecular thinning [7, 8]. Interconnections between individual trabeculae are important to maintaining the overall structure of the compartment. Greater trabecular separation compromises the integrity and strength of the bone. Sex differences are observed in trabecular arrangement which additionally contribute to the greater fracture risk in women: young women have fewer and thinner trabeculae with higher trabecular separation than young men [4, 9]. With aging, there is greater reduction in trabecular number in women than men [4, 9].
The outer cortex provides a dense outer shell, but increasing porosity and “trabecularization” reduce bone strength. At PBM, females have lower cortical porosity than males; however, this is offset by the greater CSA and cortical thickness in the males, such that strength is not compromised [4]. However, porosity increases with aging in both sexes, and this occurs at a faster rate in women than men, such that at age 80 years, cortical porosity is greater in women [4, 10].
Bone Nanoarchitecture
The importance of the collagen matrix (comprising 90 % of bone tissue) to the mechanical properties of bone is clearly demonstrated by the increased bone fragility observed in patients with osteogenesis imperfecta. This heterogeneous group of diseases illustrates that either qualitative or quantitative reductions in collagen production can lead to increased propensity to fracture. Collagen provides scaffolding on which the mineral can be deposited. The natural history of the collagen content of bone mirrors the pattern observed with bone mass: it reaches a peak in adolescence, with reduction thereafter [11]. Furthermore, cross-linkage of collagen fibrils by non-collagenous proteins confers greater tensile strength, and a reduction in the number of cross-links observed in osteoporotic bone may contribute to increased propensity to fracture [11]. In contrast, the presence of advanced glycation end products (AGE) within the bone matrix and cross-linked with collagen, which occurs as a result of high ambient glucose concentrations as seen in poorly controlled diabetes mellitus (DM), reduces the mechanical strengt h of bone and might account for the increased fracture risk of individuals with DM [11]. The crystallinity of the bone mineral within the matrix also contributes to bone strength; bones containing large hydroxyapatite crystals are more brittle and therefore more prone to fracture, whereas a greater heterogeneity in crystal size improves bone strength [12] and resistance to crack propagation.
Cellular Basis of Bone Metabolism
At the cellular level, there are three main types of bone cells: osteoblasts, osteocytes, and osteoclasts. In simple terms, osteoblasts are responsible for bone formation and may become embedded within bone mineral as mature osteocytes or remain on the surface of the bone as bone-lining cells. In contrast, multinucleated osteoclasts resorb bone. Osteoblasts and osteoclasts act in a coordinated fashion at specific sites on the surface of trabecular or cortical bone, forming “bone multicellular units” (Fig. 1.2).
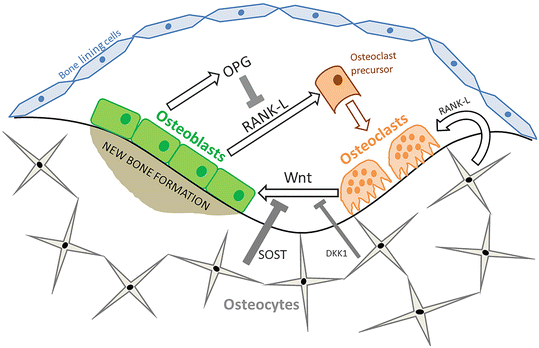

Fig. 1.2
The bone multicellular unit . The bone remodeling unit consists of osteoblasts, osteocytes, and osteoclasts, which operate in a coordinated fashion through the release of stimulatory and inhibitory cytokines. Receptor activator of nuclear factor kB ligand (RANK-L) is released from osteoblasts and osteocytes and stimulates the differentiation and activity of osteoclasts to resorb bone. RANK-L can be antagonized by osteoprotegerin (OPG). Osteoblast differentiation is stimulated by Wnt signaling from osteoclasts, while sclerostin (SOST) and Dickkopf-1 (DKK1) production by osteocytes competitively antagonizes Wnt binding to LRP5 and LRP6 receptors on the osteoblast to reduce bone formation
After new osteoid collagen matrix is laid down by osteoblasts, crystals of calcium hydroxyapatite form on the collagen fibrils, thus achieving mineralization of the bone tissue over succeeding weeks and months. Modeling is defined as the process by which bone mass is acquired during growth, repair, and adaptation to mechanical loading. In contrast, remodeling involves a cycle of resorption and formation of existing bone. Thus the balance between bone formation and resorption has a critical influence on overall bone mass and strength. During growth, formation clearly exceeds resorption, and after the achievement of PBM, the two opposing forces are in relative balance. However, as age increases into later adulthood, resorption begins to outstrip formation, and often the remodeling rate increases, reducing the opportunity for resorption cavities to be filled with new osteoid and for them to undergo a secondary mineralization. Over recent years, it has been recognized that osteocytes, which comprise 90–95 % of the cells within bone, play a key role in the regulation of these processes. The arrangement of the osteocytes within the lacunocanalicular system acts as a mechanosensory system and allows communication both directly and through the release of endocrine, paracrine, and autocrine signaling molecules to the other bone cells. There are a number of pathways which are important to the regulation of osteoblast and osteoclast activity. These are becoming increasingly recognized as targets for anti-osteoporosis agents.
Molecular Mechanisms
Osteoblasts are the primary source of receptor activator of nuclear factor κB ligand (RANK-L) , which binds to RANK on osteoprogenitor cells, stimulating the differentiation of osteoclasts and activating bone resorption [13, 14]. The production of RANK-L by osteoblasts is increased in response to disuse, estrogen deficiency, and some medications including glucocorticoids and chemotherapeutic agents [15]. The activity of RANK-L is antagonized by osteoprotegerin (OPG), which competitively binds to RANK. This prevents the binding of RANK-L to RANK, and therefore the balance of OPG and RANK-L will determine the extent of bone resorption.
The Wnt signaling pathway has a key role in osteoblast differentiation, proliferation, and bone mineralization. The activation of this pathway in osteoblasts occurs through the binding of Wnt to a membrane receptor complex comprising Frizzled (Fzd) and either low-density lipoprotein-related protein 5 (LRP5) or 6 (LRP6). This leads to cytoplasmic accumulation of β-catenin, which can subsequently increase osteoblast differentiation. There is simultaneous repression of osteoclast function through increased secretion of OPG by osteoblast and osteocytes in response to Wnt signaling. However, in remodeling, osteoclasts appear to release Wnt ligands to stimulate local differentiation of osteoblasts [16]. The importance of this pathway to bone mineralization is demonstrated clinically by the osteoporosis-pseudoglioma syndrome, which results from loss of function mutations in LRP5. Conversely, gain-of-function mutations in LRP5 can result in a high bone mass phenotype [16]. Osteocytes regulate osteoblast differentiation through secretion of sclerostin (SOST) , an antagonist of Wnt signaling which competitively binds to LRP5/6. Thus, lack of sclerostin also results in high bone mass, as observed in van Buchem disease and sclerosteosis [16]. Furthermore, osteocytes also express Dickkopf-1 (DKK1) , but to a lesser extent than sclerostin. DKK1 also antagonizes Wnt signaling and thus reduces bone mineralization. No single gene defects in DKK1 associated with alterations in bone mass have been identified, but single nucleotide polymorphism (SNP) in DKK1, in addition to other genes in the Wnt signaling pathway, has been associated with BMD in genome-wide association studies (GWAS ) [17].
Factors Influencing Bone Structural Properties
Mechanical Loading
Frost’s mechanostat theory recognizes that bone has a homeostatic mechanism which enables BMD and geometric properties of the bone to change in response to mechanical loading or strains [18]. The mechanism through which mechanotransduction of strains to a biological signal occurs is poorly understood, but is likely to involve the osteocyte. Nonetheless, the importance of mechanical loading is clearly demonstrated by deterioration in BMD in astronauts experiencing a period of weightlessness [19] and by the positive effects of exercise. Prior to the attainment of PBM, loading increases BMC and CSA [20]. These changes will confer greater bone strength and may be less marked at older ages. In the adult skeleton, mechanical loading is important for conservation of bone mass, and a recent Cochrane review concluded that exercise can reduce bone loss in postmenopausal women, although the effect size is relatively small [21]. Obese adults tend to have higher BMD than normal weight controls (although not necessarily optimally adapted for their weight), and it is likely that this is due to mechanical loading and a concomitant increase in lean mass, in addition to hormonal influences.
Hormonal Factors
Sex Steroids
Sex steroids are necessary for both linear growth during puberty and maintenance of BMD after PBM. Estrogens are essential for the pubertal growth spurt and closure of the epiphyses in both males and females, whereas the role of androgens in these processes is less clear. Androgens, in addition to estrogens, are however important for periosteal apposition which increases bone width during the pubertal growth spurt. After puberty, sex steroid concentrations affect the rate of bone remodeling, and hypogonadism results in increased osteoclast number and function [22]. Clinically, this is evident in patients with estrogen receptor deficiency and aromatase deficiency, which are monogenic disorders associated with early onset osteoporosis. Furthermore, the importance of estrogen to BMD is also indicated by the rapid increase in the incidence of fragility fractures after the menopause. Early estrogen deficiency in women particularly affects bones with high trabecular content, including vertebral bodies and the distal radius, and appears to result in a disproportionate loss of connectivity between trabeculae leading to compromised bone strength [7]. A further phase of bone loss in later years affecting both men and women also appears to affect cortical sites, with an increase in cortical porosity and reduction in cortical thickness.
Parathyroid Hormone and Phosphate Metabolism
Bone mineral acts as a reservoir for maintenance of serum calcium; its release into the serum to achieve normocalcemia is tightly regulated by parathyroid hormone (PTH) in combination with vitamin D, calcitonin, and fibroblast growth factor-23 (FGF-23). However, PTH has varied effects on bone metabolism: chronic elevations in PTH, as occurs in primary hyperparathyroidism, can result in mild reductions in BMD. Conversely, intermittent administration of low-dose PTH has anabolic effects on the skeleton [23]. This effect has been utilized in the dev elopment of PTH analogues for osteoporosis treatment and will be covered in more detail in Chap. 3.
Adipokines
There is also increasing recognition of the interaction between adipose tissue and bone mineralization, mediated through adipokines and osteocalcin. Leptin is an adipocyte-derived hormone, with key roles in regulation of appetite and body weight. However, ob/ob leptin-deficient mice have high bone mass despite hypogonadism. Furthermore, intracerebroventricular infusion of leptin reverses this high bone mass phenotype in ob/ob mice and in wild-type mice induces bone loss [22], demonstrating that leptin can influence bone metabolism. Leptin inhibits osteoblast differentiation and function via a central pathway mediated through hypothalamic activation of the sympathetic nervous system, which can stimulate β2 receptors on osteoblasts. While this reduces osteoblastic bone formation, it also increases the release of RANK-L from osteoblasts leading to increased osteoclast activity. In vitro, leptin has also been shown to directly increase osteoblast differentiation from human bone marrow stromal cells [24]. Adiponectin is also secreted by adipose tissue, but inversely to fat mass, and therefore levels are low in obesity. Adiponectin also has an effect on bone remodeling. The exact mechanisms of this action are unclear, and the balance toward formation or resorption may be dependent on the presence or absence of other factors, such as insulin and the source of adiponectin, but the adiponectin receptor has been identified on both osteoblasts and osteoclasts [25].
Epidemiology of Osteoporosis
Prevalence and Burden of Osteoporotic Fractures
In 2010, it was estimated that there were over 5.5 million men and 22 million women with osteoporosis living within the European Union (EU), representing 6.6 % and 22.1 % of the population over 50 years of age, respectively [26]. Worldwide, there are nearly 9 million osteoporotic fractures each year. In the EU, the estimated annual direct cost of 3.5 million fragility fractures in 2010 was approximately €24 billion; however, the total economic cost of osteoporosis management, including pharmacological fracture prevention and long-term fracture care, was estimated at €37 billion per year [26]. Although historically it was thought that the vast majority of this burden could be attributed to hip fracture, more recent data have suggested that just over half (54 %) of the economic cost of fracture is secondary to hip fractures, with non-hip, non-wrist, and non-spine fractures accounting for 39 %, and the remaining 7 % attributable to spine (5 %) and wrist (2 %) [26].
In addition to the economic healthcare costs, osteoporotic fractures lead to a significant individual burden. Excess mortality is a major consequence of fragility fracture, although this varies depending on fracture site and, although highest for hip fracture, has been shown to be elevated for most types of major fracture [27]. There is a five- to eightfold increase in mortality in the first 3 months following a hip fracture, and this is greater in men than women [28]. The excess risk does decrease with time, although by 10 years postfracture, it has not returned to baseline [28, 29]. Perioperative complications, including cardiovascular events, pulmonary embolism, and respiratory infections, will contribute to the increased short-term mo rtality [30], but the exact cause for the sustained increase in mortality is unclear. Data relating to the presence of prefracture comorbidities and morality rates are conflicting [29, 31]; however, postfracture frailty is likely to be a significant contributor. Furthermore, individuals who sustain one hip fracture are at higher risk of a second fracture [32, 33], and a second fracture further elevates the 5-year mortality risk [32]. Vertebral fractures are also associated with excess mortality, despite many vertebral fractures not being clinically recognized. Similarly to hip fractures, this increased risk persists for at least 5 years, although the direct fracture-related deaths are fewer. Although earlier data suggested that forearm fractures may not be associated with increased mortality [34], more recent data suggest that all major osteoporotic fractures are linked to reduced survival [27].
Functional decline is common following an osteoporotic fracture; similar to mortality rates, this is greatest for those that sustain a hip fracture, in whom only approximately two-fifths will regain their prefracture ambulatory ability at 2 years [35], and rates of admission to nursing homes in individuals following a hip fracture exceed that of non-fracturing age- and sex-matched controls [36]. Older age at fracture, malignancy, and cognitive impairment are all associated with higher risk of functional decline following a hip fracture [35]. Vertebral fractures are also associated with pain and impaired quality of life [37], whereas forearm fractures are less frequently associated with increased morbidity and demonstrate the least reduction in q uality of life [37].
Determinants of Fracture Risk
An osteoporotic bone has reduced strength, but spontaneous fractures remain rare. The epidemiology of osteoporotic fractures therefore reflects both disturbances to the biological processes involved in the balance between bone formation and resorption and also characteristics that lead to an increased propensity to fall, thus applying an impact force.
Age
There is a b imodal distribution of fractures by age; fracture incidence firstly peaks during puberty [38] and secondly in later life [39]. The peak in adolescence tends to coincide with the period of rapid skeletal growth in early to mid-puberty; these fractures are usually associated with substantial trauma and occur in the long bones. In contrast, fractures in later life are often secondary to minimal trauma.
The prevalence of osteoporosis increases significantly with advancing age after 50 years; one study reported an increased prevalence from 4.4 % of individuals aged 50–54 years to 36.8 % of those aged over 80 years [26]. Similarly, the incidence of fragility fractures increases after 50 years of age, as shown in Fig. 1.3 [40], although the absolute number of fractures plateaus in those over 80 years as the size of the at-risk population reduces [26]. The increasing incidence is in part due to age-related reductions in BMD, as the risk of fracture approximately doubles for every standard deviation decrease in BMD [41, 42], but deteriorations in cortical and trabecular structure and an the increasing incidence of falls [42] also contribute. Approximately, one-third of community-dwelling adults over 65 years will fall at least once each year [43], but the incidence is four times greater in 90-year-olds than 60-year-olds and twice as common in women than men [44]. Risk factors for falls include sarcopenia and poor functional mobility, neurocognitive impairment, poor visual acuity, disturbances to balance, cardiovascular instability, and sedative medications [44]. Similarly, the prevalence of these comorbidities tends to increas e with age (Fig. 1.3).
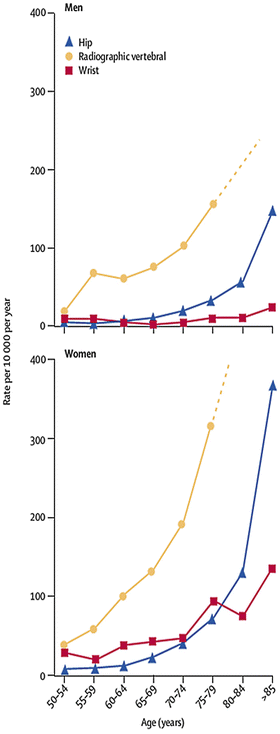

Fig. 1.3
Hip, wrist, and radiographic vertebral fracture incidence by age and gender.
From The Lancet, Vol 367 (9527), P Sambrook & C Cooper, Osteoporosis, Pages 2010–18., Copyright 2011 [40]. Reprinted with permission from Elsevier Limited
Sex
The sex distribution of fractures differs with age. In childhood, fracture rates are higher in boys [38], and this pattern remains until approximately 50–55 years of age when the sex distribution is reversed [45]. After the age of 50 years, women sustain nearly three times as many fractures as men and account for 75 % of the fracture-related costs [46]. This increase in fracture incidence in women coincides with the postmenopausal reductions in bone mass. The gender disparity is particularly marked for forearm fractures, with an age-adjusted female-to-male ratio of 4:1 due to a marked increase in forearm fractures in females with advancing age, but no simultaneous increase in males [45]. Despite the overall incidence of fractures being higher in women than men, hip fractures tend to occur at a younger age, and mortality rates are higher in males [28, 47]. This is likely to reflect higher levels of comorbidity in males than female. However, functional outcome in those that do survive hip fracture is similar between the two sexes [48].
Ethnicity
Fracture rates vary with ethnicity. A recent study of women aged over 65 years living in the USA demonstrated that the incidence of hip fracture was highest in white women, and this was 1.4, 2.0, and 2.3 times higher than that of Hispanic, Asian, and Black American women, respectively [49]. The ethnic variability in fracture rates observed in men is much lower, but white men still have a higher incidence of hip fracture [49]. Some of the variation in fracture rate is due to ethnic differences in BMD: data from the NHANES 1999–2004 cohort demonstrated that at all ages, aBMD is higher in black compared to non-Hispanic white individuals, who had greater aBMD than Mexican Americans [50]. However, the Study of Osteoporotic Fractures demonstrated that even within the same tertile of BMD, black women have a 30–40 % lower risk of fracture than white women [51], indicating that the other factors, for example, skeletal size and microarchitecture, are also likely to contribute to the fracture risk disparity. Even in childhood, African Americans have greater bone area and higher measures of bone strength than Caucasian children [52, 53], and these differences remain apparent in later life [54]. Furthermore, postmenopausal African American women also have increased trabecular thickness, cortical area, and cortical thickness compared to Caucasian women [54], which will also increase resistance to fracture.
Anthropometry, Obesity, and Body Composition
Taller stature [55, 56] and a low body mass index are well-established risk factors for fracture in postmenopausal women, whereas conversely obesity appears to be a protective factor for hip fracture in adults [57, 58]. However, the effect of obesity on the incidence of fractures across skeletal sites is not identical. In the Global Longitudinal Study of Osteoporosis in Women (GLOW), which included 46,443 women from 10 different countries, the overall incidence of fracture did not differ between obese and normal weight women, but the age of reported fracture was significantly younger in obese women than normal or underweight women [59]. Obesity was associated with a higher incidence of ankle and lower leg fractures, but lower incidence of wrist, hip, and pelvic fractures. In contrast, underweight women had a higher incidence of hip and pelvic fractures compared to normal weight and obese women [59]. This variation in fracture site by body weight may be mediated through a combination of mechanical loading resulting in a higher BMD in obese individuals and protective cushioning at some sites, but greater forces applied to other sites, in the event of a fall.
Heritable Influences
The risk of an osteoporotic fracture is greater in individuals who have a parent that suffered a hip fracture. Twin and family studies suggest that a substantial proportion of the variance in bone mass is determined by heritable factors, including intrauterine and shared environmental factors and genetic influences, although this varies by skeletal site, such that the heritance of BMD at the lumbar spine is greater than that at the wrist [60–62]. However, age also influences the heritability of BMD. The heritable component is estimated to be lower in postmenopausal compared with premenopausal women [60, 61], which most likely reflects the additional role of lifestyle-, dietary-, and disease-related factors in the latter group. Genetic determinants of femoral neck geometry [63], markers of bone turnover [64], age at menopause [65], and muscle strength [66] will also contribute to the genetic susceptibility to osteoporotic fracture.
A number of genes have now been identified as possible candidates for the regulation of bone mass and osteoporotic fracture. The majority of these influence the estrogen, Wnt signaling, or RANK-L-RANK-OPG pathways [17, 67]. However, polymorphisms at these loci explain only a small proportion (1–3 %) of the observed variance in BMD in the population, and there is increasing recognition that environmental factors might alter osteoporosis risk directly and through epigenetic mechanisms acting to influence gene expression. These gene-environment interactions might occur either in fetal development, for example, the demonstrated interaction between birth weight and the vitamin D receptor genotype in determining lumbar spine BMD [68], or in later life, for example, in the Framingham Offspring Cohort, genetic variation in the interleukin-6 promoter gene was only associated with hip BMD in a subset of women who were not using estrogen replacement and in those who had an inadequate calcium intake [69]. Over recent years, the role of epigenetic processes in mediating such interactions between environmental factors and gene expression has been increasingly recognized as fundamental to the pathogenesis of human disease [70].
Geography
There is a marked variation in hip fracture rates globally. The highest age- standardized annual hip fracture rates, observed in Scandinavia, are tenfold higher than the countries with the lowest incidence, including Tunisia, Ecuador, and Morocco [71]. Generally, hip fracture rates are highest in countries furthest from the equator, and although the underlying mechanisms remain to be elucidated, this variation may be related to differences in vitamin D status. In countries where extensive skin covering due to religious or cultural practices are the norm, such as in many of the Middle Eastern countries [71], higher rates of hip fracture are observed, which further supports lack of sunlight exposure and reduced vitamin D synthesis as contributory factors. Some migration data would suggest that this geographical variation is the result of environmental, rather than genetic, factors, although not all studies support this. Black Africans residing in the USA have higher incidence of hip fracture than Black Africans in Africa [71], but Japanese women living in Hawaii have a similar incidence of hip fracture to those who have remained in Japan [72]. Clearly any similarities and differences between the current and previous environment have to be borne in mind when interpreting such findings.
Conversely, in Sweden, the overall risk of hip fracture is significantly higher in those who were born in Sweden than for foreign-born individuals [73]. Furthermore, in the foreign-born males, the incidence of hip fracture was highest in those from Norway and Iceland and lower in individuals born in more southern European countries such as Poland and Germany [73]. A number of factors might contribute to these differences in incidence including manual labor intensity in immigrants, anthropometry, and body composition, but these results could also suggest that early life geographical factors, including vitamin D exposure, might be important to determining fracture risk.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree