Precancerous lesions
Precancerous conditions
Leukoplakia
Submucous fibrosis
Erythroplakia
Actinic keratosis
Palatal lesions in reverse smokers
Lichen planus
Discoid lupus erythematosus
Potentially malignant disorders | |
|---|---|
Leukoplakia | Oral submucous fibrosis |
Erythroplakia | Lichen planus |
Palatal lesions in reverse smokers | Actinic keratosis |
Discoid lupus erythematosus | |
Hereditary or some disorders with increased riska | |
4.3 Leukoplakia
4.3.1 Criteria and Diagnosis
4.3.1.1 Definition
In the 1978 report on the meeting for establishing the histological definition of precancerous lesions, oral leukoplakia was defined as “a white patch or plaque that cannot be characterized clinically or pathologically as any other disease” [2]. In 1984, the first international conference on oral leukoplakia was held in Malmo Sweden [3], where leukoplakia was defined as “a white patch or plaque that cannot be characterized clinically or pathologically as any other disease and is not associated with any physical or chemical causative agent except the use of tobacco” [3]. In 1994, an international symposium was held in Uppsala, Sweden, and leukoplakia was defined as “a predominantly white lesion of the oral mucosa that cannot be characterized as any other definable diseases (lesions)” [4, 5]. At a WHO workshop held in 2005, it was decided to use the term “leukoplakia” for “white plaques of questionable risk after excluding (other) known diseases or disorders carrying no increased risk for cancer” [6].
4.3.1.2 Diagnosis
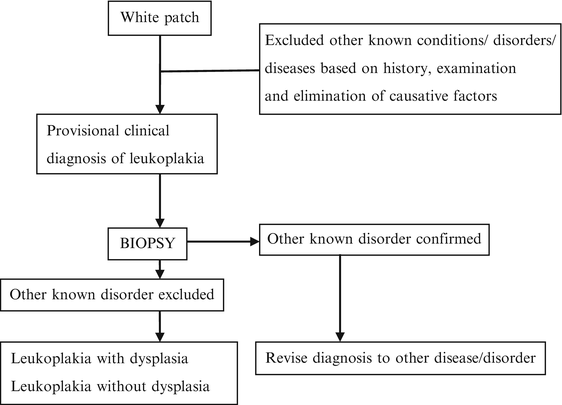
The current working group recommended the use of a three-step flowchart for the diagnosis of oral leukoplakia (Fig. 4.1) [6]. According to the flowchart, in the presence of possible etiological factors, including tobacco habits, the patient should be observed after not more than a somewhat arbitrarily chosen 2–4 weeks, which seems an acceptable period for observing possible regression after eliminating such factors. However, most leukoplakic lesions do not usually regress in such a short period, especially in smokers. Examples of white or predominantly white diseases of the oral mucosa carrying no increased risk for cancer development are listed in Table 4.3 [9].
Table 4.3
The most common white or predominantly white benign diseases of the oral mucosa and their main diagnostic criteria [9]
Lesion | Main diagnostic criteria |
|---|---|
Aspirin burn | History of local application of aspirin tablets |
Candidiasis, pseudomembranous | Clinical aspect (pseudomembranes, often of symmetrical pattern) |
Candidiasis, hyperplasticaa | |
Frictional lesion | Presence of mechanical irritation (e.g., habit of vigorous toothbrushing) |
Hairy leukoplakia | Clinical aspect (incl. bilateral localization on the tongue); histopathology (incl. EBV) |
Leukoedema | Clinical aspect (incl. symmetrical pattern) |
Linea alba | Clinical aspect (incl. location on the line of occlusion in the cheek mucosa) |
Lupus erythematosus | History of skin lesions; clinical appearance (incl. bilateral pattern); histopathology |
Morsicatio (habitual chewing or biting of the cheek, tongue, lips) | History of habitual chewing or biting; clinical aspects |
Papilloma and allied lesions | Clinical aspect; histopathology |
Syphilis, secondary (“mucous patches”) | Clinical aspect: histopathology |
T. pallidum; serology | |
Tobacco-induced lesionsb | |
Smoker’s palate (nicotinic stomatitis) | Clinical aspect; history of smoking |
Snuff induced lesion | Clinical aspect; site were snuff isplaced |
White sponge nevus | Family history; clinical aspect (often symmetrical pattern) |
van der Waal [9] proposed that the term leukoplakia can be used at different levels of certainty (C-factor) as a clinical term (C1, diagnosed from a single visit, or C2, diagnosed by a negative results of elimination of etiologic factors) or a clinicopathological term (C3, confirmed pathologically on incisional biopsy, or C4, confirmed pathologically in a resected specimen) [9]. Amagasa et al. recommended biopsy for the accurate diagnosis of leukoplakia, especially for investigating the malignant transformation of leukoplakia, which is in agreement with other studies on the malignant transformation of leukoplakia [10].
4.3.2 Clinical Aspects
4.3.2.1 Sex
With regard to the sex of patients with leukoplakia, many studies have reported a predominance of oral leukoplakia in men [11–24], with an extreme predominance in men in India and other countries where tobacco chewing and/or smoking habits are prevalent [19, 25–27]. On the contrary, some studies have reported an equal male-to-female ratio or a slight female predominance in the United States, other Western countries, and Japan [28–31].
4.3.2.2 Age
4.3.2.3 Site
With regard to the site of oral leukoplakia, many studies have reported the buccal mucosa as a common site [12, 13, 16, 24, 25, 30]. In India and other countries with tobacco chewing and/or smoking habits, the vast majority of leukoplakia cases were located on the buccal mucosa and labial commissure, with a few on the tongue or floor of mouth [20, 26, 27]. In Japan, however, the lower gum/alveolar ridge and the tongue were the most frequent sites followed by the buccal mucosa and upper gum/alveolar ridge [10], which is partially in agreement with other studies conducted in Japan [31, 34]. In the Netherlands, the tongue and floor of mouth were reported as the most frequently affected sites [29]. The sites commonly affected by oral leukoplakia may be the result of oral habits, especially tobacco smoking and/or various unknown causes.
4.3.2.4 Clinical Features and Types
Pindborg et al. [35] described in 1963 that speckled leukoplakia is often associated with epithelial dysplasia and carcinoma, and other researchers have reported their clinical types of oral leukoplakia with epithelial dysplasia and malignant transformation [12, 20, 29, 36]. In 1978, leukoplakia was divided into two main types: homogeneous and non-homogeneous [2]. Homogeneous leukoplakia was subclassified into flat, corrugated, wrinkled, and pumice-like, while non-homogeneous leukoplakia was subclassified into verrucous, nodular, ulcerated, and erythroleukoplakia (Table 4.4); these classifications were recognized by the WHO in 1994 [4, 5].
Homogeneous | Nonhomogeneous |
|---|---|
Flat | Verrucous |
Corrugated | Nodular |
Wrinkled | Ulcerated |
Pumice-like | Erythroleukoplakia |
At a WHO workshop held in 2005, the working group noted that nonhomogeneous varieties include speckled, nodular, and verrucous types; however, the working group consensus was that broadly dividing leukoplakia into homogeneous or non-homogeneous categories was imprecise and of limited value. Nevertheless, patients with mixed white and red plaques should be recognized as having a higher risk status, and such cases should be denoted as “erythroleukoplakia” [6]. Proliferative verrucous leukoplakia (PVL), which was reported in 1984 by Hansen et al. [37], was also recognized at the workshop. This form of leukoplakia is characterized by multiple, simultaneous leukoplakias as the disease is visibly multifocal and frequently presents across a wide area of the oral mucosa with most patients developing OSCC over long follow-up periods [37, 38]. Unfortunately, this type of leukoplakia cannot be determined in the initial stage and is diagnosed during or after its course.
4.3.3 Histopathological Aspects
4.3.3.1 Epithelial Dysplasia
It is largely accepted worldwide that a general disturbance of the epithelium is designated as “dysplasia” and that the potential for developing invasive carcinoma increases with the severity of dysplasia [5, 39]. Epithelial dysplasia is conventionally classified into mild, moderate, and severe including carcinoma in situ (CIS). Many reports have attempted to show a typical sample for each degree of epithelial dysplasia [5, 39, 40]. However, the degree of epithelial dysplasia is difficult to diagnose and several studies have reported great interexaminer and intraexaminer variability when assessing the presence or absence, as well as the grade, of oral epithelial dysplasia [41–43]. To increase the likelihood of agreement between oral pathologists by reducing the number of choices, a two-tier classification system (no/questionable/mild denoting low risk and moderate/severe denoting high risk) was considered at the 2005 Oral Cancer and Precancer Workshop coordinated by the WHO, with samples of each of the three grades of epithelial dysplasia [44].
The book World Health Organization Classification of Tumours (2005) recommended using the new concept of “epithelial precursor lesions,” which were previously termed “premalignant lesions,” and the terms “squamous intraepithelial neoplasia” and “squamous intraepithelial lesions” according to the Ljubljana Classification instead of “epithelial dysplasia” [45]. Although this classification was reported to be useful for predicting the malignant transformation of the hypopharynx and larynx [45–49], the WHO consensus group did not favor its use for oral mucosal lesions [44]. In addition, the current working group [6] did not actively accept the term “epithelial precursor lesions”, but adopted the new term “potentially malignant disorders.”
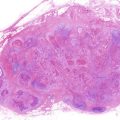
CIS of the oral mucosa is defined in the WHO classification as histologic changes involving the full thickness or almost the full thickness of squamous epithelium showing cellular features of carcinoma without stromal invasion [5, 39, 40, 50] and is in agreement with the definition proposed by the current working group [44]. Typically, most CIS of the upper aero digestive tract are nonkeratinizing, although keratinizing CIS can be encountered in the vocal cords or oral cavity [51]. Lesions with similar histological figures in the esophagus are considered to be squamous cell CIS (noninvasive squamous cell carcinoma) by Japanese pathologists and high-grade intraepithelial neoplasia by Western pathologists [52]. Izumo [53] in his review article noted that CIS of the oral mucosa show superficial maturation and differentiation. Some recent clinicopathological examinations in Japan have also revealed several variations of CIS of the oral mucosa [54] that are not included in the WHO classification [44, 45]. In the future, the suitability of the CIS criteria proposed by Japanese oral pathologists will be evaluated.
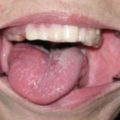
In relation between the clinical classification and epithelial dysplasia, Sugár and Bánóczy [55] reported in their three-tiered clinical classification in 1969 that leukoplakia erosiva and leukoplakia verrucosa were more often associated than leukoplakia simplex with epithelial dysplasia. In 1977, Amagasa et al. [56] developed a clinical classification system for oral leukoplakia based on lesions with or without elevation and with or without red components that was further developed in 2006 [10]. In this system, oral leukoplakia was classified into four clinical types: type I, a flat white patch or plaque without red components; type II, a flat white patch or plaque with red components; type III, a slightly raised or elevated white plaque; and type IV, a markedly raised or elevated white plaque (Table 4.5, Fig. 4.2a–d). With regard to epithelial dysplasia (Fig. 4.3a–c) in our classification system, type II leukoplakia was found to be significantly associated with epithelial dysplasia (Table 4.6) [10].
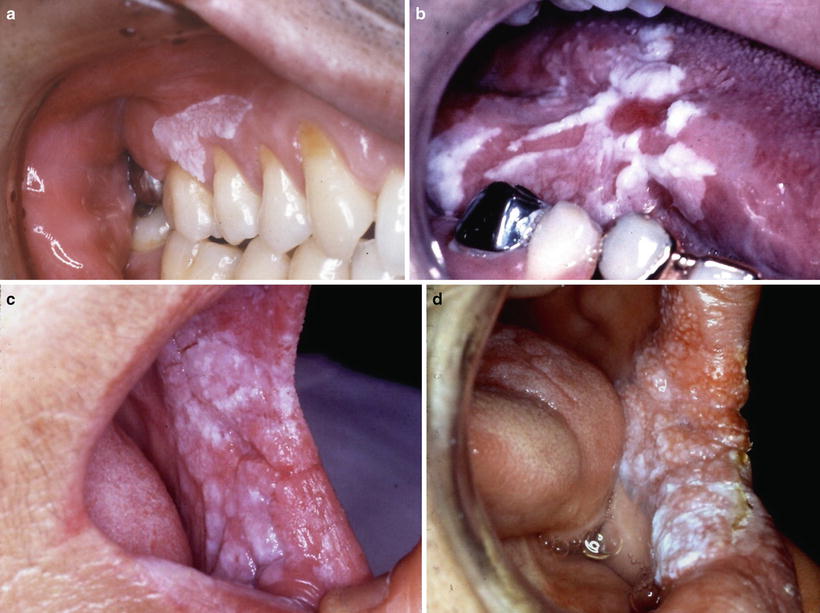
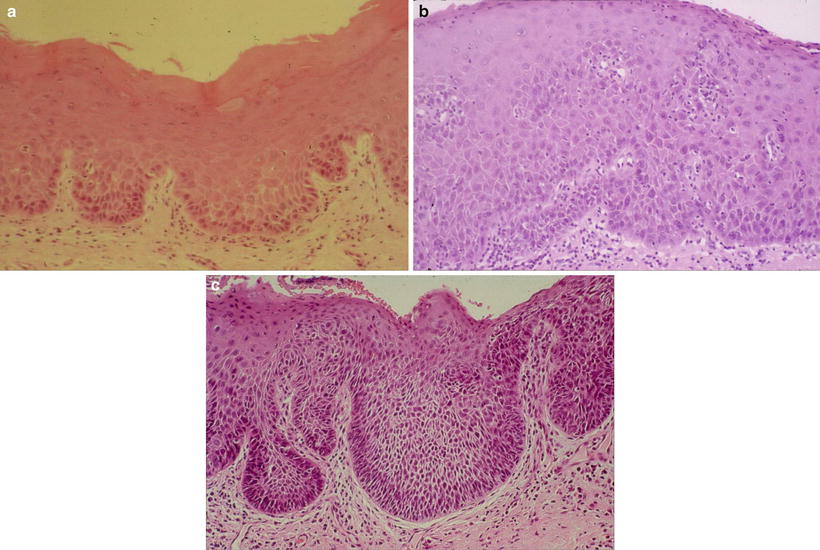

Fig. 4.2
Clinical features of leukoplakia can be classified into four types: type I (a) flat white patch on the of the maxillary gingival, type II (b) white patch and plaque with erosion on the tongue, type III (c) slightly elevated white plaque on the buccal mucosa, and type IV (d) markedly elevated white plaque with partly granular appearance on the buccal mucosa [10]

Fig. 4.3
Histopathological features of leukoplakia. (a) Mild dysplasia: slightly irregular epithelial architecture limited to the lower third of the epithelium. Basal–parabasal cells show hyperchromatic large nuclei and loss of polarity. (b) Moderate dysplasia: hyperplastic epithelium with increased number of polymorphic cells extends up to two-thirds of the epithelium, and cellular atypia is more pronounced with nuclear pleomorphism. (c) Severe dysplasia: the atypical basal-like and spinous-like cells occupy almost all epithelial layers. Squamous epithelium is of variable thickness with drop-shaped rete ridges and prominent cytologic atypia [10]
Type I | Flat, white patch/plaque without red components |
Type II | Flat, white patch/plaque with erosion or red components |
Type III | Slightly raised or elevated white patch/plaque |
Type IV | Markedly raised or elevated white patch/plaque |
Type | Cases | No dysplasia | Dysplasia | Total number of dysplasia (%) | ||
|---|---|---|---|---|---|---|
Mild | Moderate | Severe | ||||
I | 328 | 203 | 86 | 30 | 9 | 125 (38.1)* |
II | 71 | 13 | 31 | 16 | 11 | 58 (81.7)* |
III | 24 | 11 | 10 | 2 | 1 | 13 (54.2)* |
IV | 21 | 7 | 11 | 1 | 2 | 14 (66.7)* |
Total (%) | 444 | 234 (52.7) | 138 (31.1) | 49 (11.0) | 23 (5.2) | 210 (47.3) |
4.3.3.2 Diagnosis of Epithelial Dysplasia with a Colorimeter and Iodine Staining
Biopsy or a surgical procedure should always be performed for the diagnosis of epithelial dysplasia. However, diagnosis of this disease by a noninvasive procedure is desirable. The efficacy of vital iodine staining for the diagnosis of cancer or dysplasia has been reported in some papers [57–60]. In particular, Maeda et al. attempted to use a colorimeter after iodine staining to clarify the relationship between lesion color (i.e., the color of the unstained lesion) and histopathological findings after staining (Fig. 4.4a, b) [61] and found a significant color difference between lesions with moderate to severe dysplasia and those with mild to no epithelial dysplasia. Colorimetry with iodine staining for premalignant lesions may be useful for distinguishing moderate to severe dysplasia from mild to no dysplasia before biopsy. However, this combination method for PMD lesions is only suitable in the two-tier pathological classification system proposed by the working group.
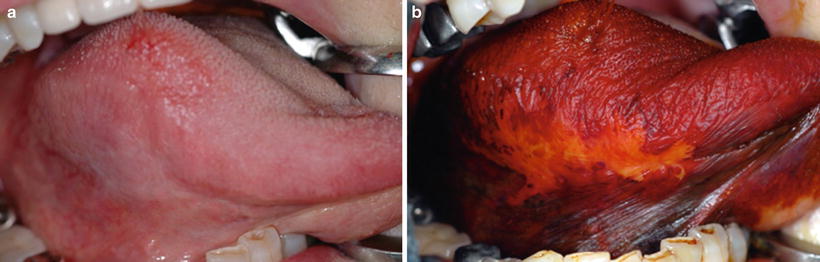

Fig. 4.4
Leukoplakia on the lateral border of tongue, showing dull border before 3 % iodine staining (a) and showing a clear border after staining (b) [87]
Maeda et al. also measured the deviation of the macroscopic and histopathological boundary of oral leukoplakia after iodine staining. The histopathological boundary of the lesions with epithelial dysplasia was at most 4.36 mm beyond the macroscopic boundary, suggesting that PMDs should be surgically removed with a safety margin >5 mm after iodine staining [62].
4.3.4 Prognosis and Development of Oral Squamous Cell Carcinoma
4.3.4.1 Prognosis
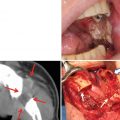
Development of OSCC in patients with oral leukoplakia is a serious problem; however, knowing the natural history of oral leukoplakia is also important for both aggressive and conservative treatment of oral leukoplakia (Fig. 4.5a–e). Mehta et al. reported that 42.5 % of untreated leukoplakias disappeared within 5 years and 45.3 % within 10 years in persons who chewed tobacco, whereas 41.5 % of untreated lesions remained unchanged in 5 years and 31.6 % in 10 years in smokers in India [20]. During follow-up of patients in India, approximately 30–40 % of oral leukoplakias had decreased in size or disappeared, 30–60 % remained unchanged, and 11 % had increased in size [18–20], and regression was more frequently observed in tobacco chewers and pipe smokers than in those who smoked cigarettes [63].
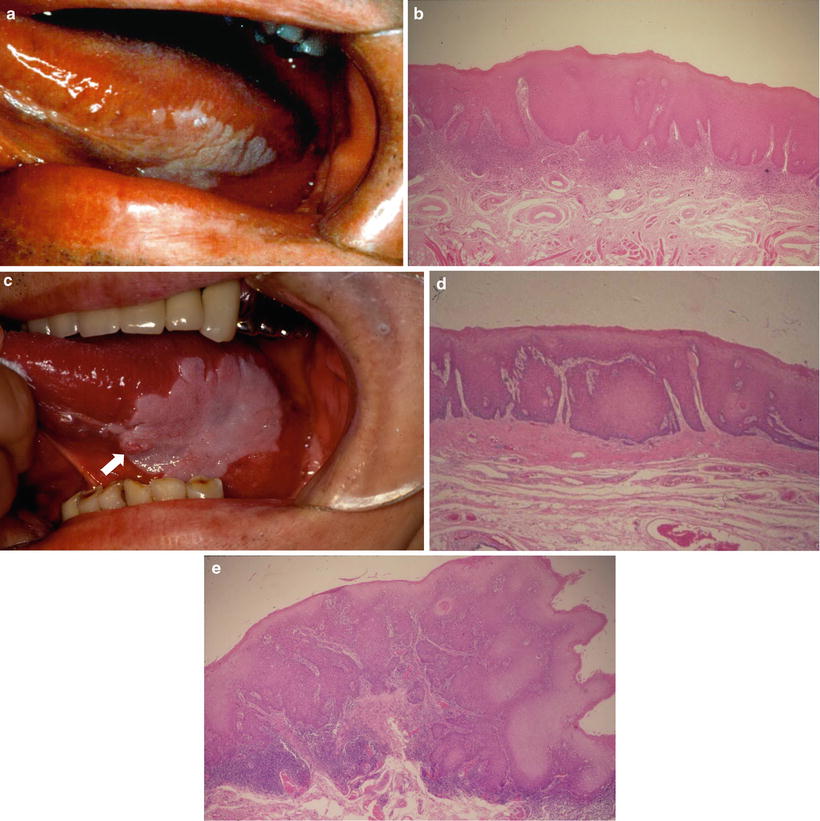

Fig. 4.5
Leukoplakia on the tongue showing a flat white plaque (a) and a biopsy specimen of the leukoplakia showing hyperplasia with moderate epithelial dysplasia (b). Seven years later without any treatment, a small-red-exophytic growth developed at the white plaque (↑) (c), a resected material specimen showing sever epithelial dysplasia (d) with early invasive squamous carcinoma (e) [87]
On the other hand, during follow-up periods of 1–10 years for 248 patients with leukoplakia in Denmark, lesions disappeared in 43 (20.1 %), decreased in size in 38 (17.8 %), and increased in size in 7 (3.3 %) [36]. Silverman reported that lesions in patients in the United States decreased or disappeared in 49 of 133 current smokers (37 %), in 22 of 50 past smokers (44 %), and in only 2 of 74 nonsmokers (3 %) [28]. Including the above reports, approximately 20–37 % of lesions completely disappeared after eliminating the causative factors, including a reduction in tobacco consumption or abstinence; 20–50 % or more decreased in size; and only 10 % or less increased in size in developed countries [28, 64, 65]. However, similar decreases in the number or size of oral leukoplakias were not observed in Japan during follow-up [10, 11].
4.3.4.2 Development of Oral Squamous Cell Carcinoma
Malignant Transformation Rate and Cumulative Malignant Transformation Rate
Many studies have reported so-called malignant transformation rates of oral leukoplakia ranging from 0.13 to 17.5 % (Table 4.7) [10, 12, 18–22, 28, 29, 32, 36, 66–68]. Among these reports, a community-based survey in India revealed a malignant transformation rate of oral leukoplakia of 0.13–2.2 %. However, the rate of oral leukoplakia derived from studies of hospital-based samples was 3.6–17.5 %, showing a higher incidence of malignant transformation than in India. The rate of malignant transformation may increase when patients are followed over a longer term and may decrease when patients are lost early to follow up. The resulting rate of malignant transformation may therefore be unreliable. To eliminate this bias, calculation of the cumulative survival rate is necessary to determine the cumulative malignant transformation rate. The cumulative transformation rates reported by some researchers are shown in Table 4.8; the 5-year cumulative malignant transformation rate ranged from 1.2 to 14.5 % [29, 31, 32, 34, 69, 70].
Authors [references] | Country | Year | Number of patients | Malignant transformation (%) | Observation periods (mean) years |
|---|---|---|---|---|---|
Silverman et al. [18] | India | 1976 | 4,762 | 0.13 | 2 |
Gupta et al. [19] | India | 1980 | 360 | 0.3 | 1–10 (7) |
Mehta et al. [20] | India | 1972 | 117 | 0.8 | 10 |
Gupta et al. [19] | India | 1980 | 410 | 2.2 | 1–10 (8) |
Roed-Peterson et al. [66] | Denmark | 1971 | 331 | 3.6 | 1 ≦ (4.3) |
Einhorn et al. [32] | Sweden | 1967 | 782 | 4.0 | 1–20 |
Pindborg et al. [36] | Denmark | 1968 | 248 | 4.4 | 1–9 |
Kramer et al. [67] | England | 1969 | 187 | 4.8 | 1–16 |
Silverman et al. [21] | USA | 1968 | 117 | 6.0 | 1–11 |
Banocy et al. [12] | Hungary | 1977 | 670 | 6.0 | 1–30 |
Amagasa et al. [10] | Japan | 2006 | 444 | 7.9 | 1–29 |
Lind [68] | Norway | 1987 | 157 | 8.9 | 6≦ |
Gangadharan et al. [22] | India | 1971 | 626 | 10.0 | 1–19 |
Schepman et al. [29] | Holland | 1998 | 166 | 12.0 | 6 months–17 (2.7) |
Silverman et al. [28] | USA | 1984 | 257 | 17.5 | 6 months–39 (7.2) |
Authors (year) | Country | Number of patients | 5-year (%) | 10-year (%) | Follow-up period (mean) |
|---|---|---|---|---|---|
Einhorn et al.[32] | USA | 782 | 1.6 | 2.4 | 1–20 years |
Amagasa et al. [69] | Japan | 169 | 12.0 | 21.0 | 1–20 years |
Inoue et al. [70] | Japan | 75 | 4.5 | 9.7 | 7 months–26 years (12 years 8 months) |
Kirita et al.[31] | Japan | 102 | 1.2 | 6.6 | 7 months–12 years 1 months (6 years 2 months) |
Schepman et al. [29] | Netherlands | 166 | 14.5 | 29.0 | 6 months–16 years 9 months [6 years 2 months] |
Kawabe et al. [34] | Japan | 237 | 4.7 | 11.1 | (56.5 ± 43.4 months) |
[median] |
4.3.4.3 Sex and Malignant Transformation
With regard to the relationship between sex and malignant transformation, of 331 patients with leukoplakia in Denmark, 5.8 % of women developed OSCC in contrast to 2.1 % of men (2.8-fold higher in women) [66]. In the United States, an equal sex ratio was found for leukoplakia (125 men vs. 132 women) with slightly more women having cancer (26 women vs. 19 men). Therefore, the rate of malignant change in women was 1.3-fold higher than in men [28]. In Japan, of 444 patients with oral leukoplakia, 17 of 275 men (6.2 %) and 18 of 169 women (10.7 %) developed OSSC (1.7-fold higher in women) [10]. Reports on the malignant transformation of oral leukoplakia in developed countries showed relatively more frequent development of OSCC from leukoplakia in women than in men [18, 66].
Lind [68] reported that women were more likely to undergo malignant transformation than men and that epithelial dysplasia was more prevalent in women. This was further supported by the findings of other authors [12, 21, 31, 36]. The following risk factors for malignant transformation were listed by Schepman et al. [29]: female sex, absence of smoking habits in women (p < 0.05), and nonhomogeneous clinical aspects (p < 0.01).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree