Chapter 66 Ophthalmologic Disease
Ocular manifestations are common in patients with AIDS, and in the era before highly active antiretroviral therapy (HAART), the majority of patients with AIDS developed some form of ocular involvement during the course of their disease (Table 66-1).1 The most frequently encountered ocular manifestation was microangiopathy, most often recognized in the retina and often referred to as ‘AIDS retinopathy’, ‘HIV retinopathy’, or ‘noninfectious HIV retinopathy’. Opportunistic ocular infections, particularly cytomegalovirus (CMV) retinitis, were common and a substantial cause of visual morbidity. Ocular structures also may be affected by those neoplasms seen in patients with AIDS, such as Kaposi sarcoma or lymphoma, by neuroophthalmic lesions, or by adverse drug side effects.
Table 66-1 Frequencies of Ocular Manifestations of AIDS in the Pre-HAART Era
| Lesion | Frequency (%) |
|---|---|
| Microangiopathy | |
| Conjunctival | 75 |
| Retinal | 50–67 |
| Opportunistic ocular infections | |
| Cytomegalovirus retinitis | 30 |
| Varicella-zoster virus (VZV) | |
| Herpes zoster ophthalmicus | 3–4 |
| VZV retinitis | <1 |
| Toxoplasmic retinitis | 1–3 |
| Pneumocystis choroidopathy | <1 |
| Microsporidial keratitis | <1 |
| Ocular syphilis | <1 |
| Ocular neoplasms | |
| Kaposi sarcoma (lids, conjunctiva) | 1–4 |
| Lymphoma (orbital, intraocular) | <1 |
| Neuroophthalmic lesions | 5–10 |
OCULAR MICROANGIOPATHY
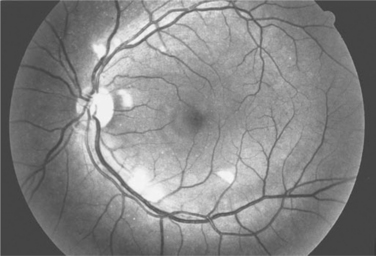
Ocular microangiopathy is the most common ophthalmologic finding in patients with AIDS.1–8 The most often recognized manifestation of this microangiopathy is retinopathy, consisting of cotton wool spots (Fig. 66-1) and less frequently intraretinal hemorrhages.1–5 Cotton wool spots are microinfarcts of the nerve fiber layer of the retina and are due to occlusion of the retinal capillaries. In the pre-HAART era retinal microangiopathy was recognized clinically in about one-half of patients with AIDS but substantially less frequently in earlier stages of HIV infection.1,5 Clinically evident HIV retinopathy is associated with low CD4+ T-lymphocyte counts, particularly <100 cells/μL.6,7 Fluorescein angiographic studies and autopsy studies3,4 have suggested that there was an even higher frequency of microangiopathic changes. Although conjunctival changes were reported less frequently, one study suggested that conjunctival vascular changes also were common in patients with AIDS.8
The pathogenesis of ocular microangiopathy is unknown; hypotheses have included (1) circulating immune complex disease,2–4 (2) infection of the retinal vasculature by HIV,9 and (3) hematological abnormalities.10 Polyclonal B-cell activation is present in patients with AIDS,11 circulating immune complexes have been reported,2,12 and immunoglobulin deposition has been demonstrated within the retinal capillaries,4 suggesting that immune complex deposition disease may account for the microangiopathy.3 Alternatively, HIV infection of the retinal vascular endothelial cells has been demonstrated.9 However, some authors have argued that the amount of HIV infection of the retinal vascular endothelial cells is inadequate to account for the retinal vasculopathy.13
HIV retinopathy generally is clinically silent.1 Occasional patients will have larger vessel retinal disease, such as a branch vein occlusion or central retinal vein occlusion.1,14 Patients with HIV infection and without infectious retinitis have been reported to have abnormalities in subjective measures such as visual acuity, visual field, and contrast sensitivity and in objective measures such as optical coherence tomography and electrophysiological testing.15–20 Autopsy studies have reported loss of optic nerve fibers.21 It has been speculated that this loss of optic nerve fibers may be due to either a cumulative insult from the microinfarcts of the nerve fiber or a direct HIV-related toxic effect on the optic nerve.
CYTOMEGALOVIRUS RETINITIS
Epidemiology
Disease caused by CMV is among the most common opportunistic infections in patients with AIDS.22,23 In the era before HAART, CMV disease affected an estimated 45% of patients.23 Infection of the retina with CMV accounted for 75–85% of all CMV disease,24,25 and it was estimated that 30% of patients with AIDS would develop CMV retinitis sometime between the diagnosis of AIDS and death.25 CMV retinitis is a late-stage manifestation of AIDS, typically associated with CD4+ T-lymphocyte counts <50 cells/μL.24–26 The incidence of CMV retinitis among patients with CD4+ T-lymphocyte counts <100 cells/μL was ∼10% per year prior to the widespread use of HAART, and that among patients with a CD4+ T-lymphocyte count <50 cells/μL, it was ∼20% per year.24,26 HAART has resulted in a 55–95% decrease in the numbers of new cases of CMV retinitis at major urban medical centers throughout the United States since 1995.27–29 This decline is due to a decrease in the cohort of patients with low CD4+ T-lymphocyte counts and low viral loads that is, those patients who are at risk for CMV retinitis and has occurred as a result of the improved immune function seen in patients treated with HAART. A similar, but much more modest and short-lived, drop in the incidence of CMV retinitis was seen when zidovudine was first introduced,27 an observation consistent with this premise and the more modest effect of monotherapy with zidovudine on HIV. Recent data suggest that the incidence of CMV retinitis now has stabilized at ∼20% of that in the era prior to HAART and that the majority of new cases are seen either in patients who are HAART-naive or who have failed at least one HAART regimen.30–32
Diagnosis and Natural History
CMV retinitis can be diagnosed reliably by an experienced ophthalmologist based on its clinical appearance (Fig. 66-2). CMV retinitis typically is described as a focal necrotizing retinitis, which may or may not be hemorrhagic. Unless there is immune reconstitution, untreated CMV retinitis spreads throughout the retina over a period of a few months, resulting in total retinal destruction and irreversible blindness.32 The symptoms of CMV retinitis are nonspecific and include floaters, flashing lights, loss of visual field, or a vague sense of visual loss. CMV retinitis often is asymptomatic, and two studies estimated the prevalence of asymptomatic and undiagnosed CMV retinitis at 13–15% of patients with CD4+ T-lymphocyte counts <50 cells/μL.7,34 This fact led some experts to recommend routine evaluation of patients at high risk for CMV retinitis by an ophthalmologist using dilated indirect ophthalmoscopy in order to detect asymptomatic CMV retinitis. Although not universally accepted, and although no long-term outcome studies have been performed to validate this approach, a typical recommendation was that patients with CD4+ T-lymphocyte counts <50 cells/μL be seen every 3–4 months. Patients with CD4+ T-lymphocyte counts >100 cells/μL are at a low risk for the development of CMV retinitis and generally are seen annually. Patients with CD4+ T-lymphocyte counts between 50 and 100 cells/μL are often seen twice annually. Ophthalmoscopy using the direct ophthalmoscope through an undilated pupil evaluates only 10% of the retinal area and is inadequate for either the diagnosis or the routine evaluation of patients for CMV retinitis.
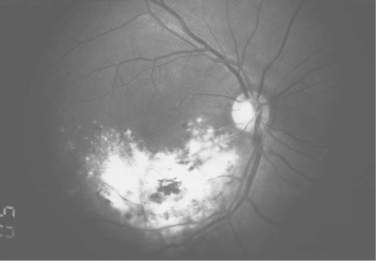
Figure 66-2 CMV retinitis in a patient with AIDS.
From Jabs DA. Ocular manifestations of HIV infection. Trans Am Ophthalmol Soc 93:623, 1995.
Treatment
The goal of treatment of CMV retinitis is to arrest progression of the disease, prevent further spread of infection in the retina, and preserve visual function. Treatment with anti-CMV agents suppresses viral replication in the eye but does not eliminate the virus.35 Discontinuation of therapy in patients who remain immunocompromised is associated with prompt relapse of the retinitis.36 Each relapse of retinitis is associated with retinal destruction and loss of visual field. Prior to the availability of HAART, the median time to bilateral visual acuity of 20/50 or worse (‘visual impairment’) was 16 months, and the median time to bilateral visual acuity of 20/200 or worse (‘legal blindness’) was 21 months.1 Therefore, unless there is immune recovery from antiretroviral therapy (defined as an increase in CD4+ T-lymphocyte count to >100 cells/μL), long-term suppressive anti-CMV therapy is required.
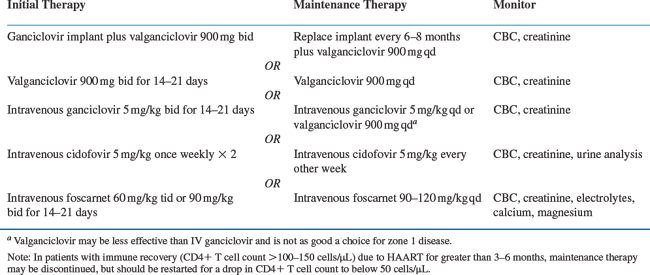
Treatment options for CMV retinitis are outlined in Table 66-2. When systemically administered anti-CMV drugs are used, the treatment of CMV retinitis generally is performed in a two-step fashion. Initially a high dose of an anti-CMV drug is given to control the infection (induction), and subsequently a lower dose is given to prevent relapse (maintenance therapy or secondary prophylaxis). Four drugs have been approved by the US Food and Drug Administration (FDA) for the treatment of CMV retinitis: ganciclovir (Cytovene), foscarnet (Foscavir), cidofovir (Vistide), and fomivirsen (Vitravene). Ganciclovir is available both as an intravenous and oral formation and as a surgically placed slow-release implant (Vitrasert). A pro-drug of ganciclovir, valganciclovir (Valcyte), has replaced the use of oral ganciclovir because its bioavailability is similar to that of IV ganciclovir.37 Foscarnet and cidofovir are available only as intravenous formulations. Intravenous ganciclovir and intravenous foscarnet therapy require twice-daily intravenous infusions for induction therapy and once-daily intravenous infusion for maintenance therapy. Both require the placement of a permanent indwelling central venous catheter. Fomivirsen is available for intravitreous injection only, but currently is not being produced.38 The intravenous formulation of ganciclovir, foscarnet, and cidofovir may be used for intravitreous injections as well. Intravenous ganciclovir and foscarnet therapy require twice-daily infusions for induction therapy and once-daily infusions for maintenance therapy. Both require the placement of a permanent indwelling central venous catheter. Because cidofovir is given once weekly for induction and once every other week for maintenance therapy, a permanent indwelling catheter is not required.
Despite the use of chronic maintenance therapy with systemically administered drugs, relapse of CMV retinitis in patients without immune recovery is typical and occurs in almost all such patients given sufficient time.1 Therefore, the efficacy of an anti-CMV drug has been evaluated by its ability to prolong the time to relapse, typically defined as the time to progression. Progression is the movement of the border of a CMV lesion a specified distance, typically 750 μm, along a front of 750 μm, or the development of a new CMV lesion, one quarter of the size of the optic disc.39,40 The comparative efficacy of two drugs is determined by their relative ability to prolong the time to progression.40 The efficacy of a new anti-CMV drug is evaluated by comparing the ability of that drug to prolong the time to progression versus observation. This approach has been used to demonstrate the efficacy of intravenous ganciclovir, intravenous foscarnet, intra-venous cidofovir, the ganciclovir implant, and intravitreous fomivirsen.38,41–45
The posterior pole of the retina (also referred to as the macula) contains the vital ocular structures, the optic nerve and fovea, responsible for good reading acuity. Lesions adjacent to the optic nerve or fovea are immediately vision threatening. For clinical trial purposes, the retina has been arbitrarily divided into three zones. Zone 1 encompasses a region within 3000 μm from the center of the fovea and 1500 μm from the edge of the optic nerve. Lesions in zone 1 are immediately vision threatening. Zone 2 goes from the edge of zone 1 to the equator of the eye, and zone 3 extends anteriorly from the equator to the pars plana of the retina. Lesions in zones 2 and 3 become vision threatening given sufficient time but are not immediately vision threatening. Therefore, patients with small ‘peripheral’ lesions located entirely in zone 2 or 3 may be observed for a short period of time without increased risk of loss of visual acuity.43
Ganciclovir
Ganciclovir was the first drug approved by the FDA for the treatment of CMV retinitis in immunocompromised patients.1,33,39,41 It is a nucleoside analog, which is taken up by the virally infected cells, triphosphorylated, and inhibits viral replication through its effect on the viral DNA polymerase. The first phosphorylation step is performed by a virally encoded phosphotransferase, whereas the next two phos-phorylation steps are performed by cellular enzymes. Ganciclovir induction is given for 2–3 weeks at a dose of 5 mg/kg every 12 h. Maintenance therapy is given at 5 mg/kg intravenously once daily. Ganciclovir has been demonstrated to be effective in the treatment of CMV retinitis in controlled studies.33,41 The most frequently encountered side effect of ganciclovir is a reversible granulocytopenia. An absolute neutrophil count of <500 cells/μL occurs in approximately one-third of patients treated for 6 months with intravenous ganciclovir.46 Granulocytopenia promptly reverses with discontinuation of the drug and typically is treated with hematologic growth factors, such as granulocyte colony-stimulating factor (filgrastim, Neupogen).47 Clinically significant thrombocytopenia occurs in less than 10% of patients and is reversible with discontinuation of the drug. Maintenance therapy with IV ganciclovir requires the placement of a permanent indwelling catheter. The rate of complications from the indwelling catheter, including catheter-related infections, has been reported to be 0.44/person-years (PY) among patients with AIDS and CMV retinitis.40,48
An oral formulation of ganciclovir was used for maintenance therapy after induction with intravenous ganciclovir, but has lost favor due to its limited absorption from the gastrointestinal tract,49 which makes it less effective than IV ganciclovir as maintenance therapy.50 An oral ganciclovir prodrug, valganciclovir, appears to produce blood levels similar to that of IV ganciclovir.37 The induction dose of valganciclovir is 900 mg twice daily, and the maintenance dose is 900 mg once daily.
Foscarnet
Foscarnet is a pyrophosphate analog that inhibits the CMV DNA polymerase. The induction dose of foscarnet is 90 mg/kg twice daily and the maintenance dose is 90 to 120 mg/kg once daily.52 A randomized controlled clinical trial demonstrated that foscarnet is effective for controlling CMV retinitis,42 and the Foscarnet–Ganciclovir Cytomegalovirus Retinitis Trial demonstrated that intravenous ganciclovir and intravenous foscarnet were equivalent for controlling CMV retinitis.53 The most important side effect of foscarnet is reversible nephrotoxicity, which occurs in ∼13% of patients treated for 6 months.46 The serum creatinine and electrolytes (including potassium, calcium, and magnesium) must be monitored twice weekly during induction therapy and once weekly during maintenance therapy, and frequent dosage adjustments often are required. Other important side effects include metabolic abnormalities, and potassium, magnesium, and calcium supplementation often are required.46 Much less common side effects include genital ulcers and infusion-related nausea.46
Cidofovir
Cidofovir is a nucleotide analog, and its intracellular activation requires two phosphorylation steps, which are mediated by cellular enzymes. Cidofovir has a prolonged duration of effect and can be given as an intermittent intravenous infusion. Cidofovir induction is given as 5 mg/kg once weekly for 2 weeks, and maintenance therapy is given once every 2 weeks at a 5 mg/kg dose. Because of its potential nephrotoxicity, cidofovir is given in conjunction with probenecid and saline hydration.54 Two studies comparing cidofovir to observation in patients with small peripheral CMV lesions have demonstrated that cidofovir is effective for the treatment of CMV retinitis.43,44 Cidofovir’s major side effect is nephrotoxicity, which is not always reversible.53 Because protein-uria appears to predate a rise in serum creatinine, all patients are monitored for proteinuria, as well as serum creatinine, prior to each cidofovir infusion. Persistent proteinuria of 3+ or greater that does not clear with hydration is an indication for discontinuation of cidofovir therapy. Proteinuria of 2+ or more occurs at a rate of 1.22/PY. Other indications for discontinuing cidofovir include a serum creatinine of 2.0 mg/dL or greater or a rise in the serum creatinine by 0.5 mg/dL or greater. Withdrawal of cidofovir resolves the proteinuria in 90% of cases.55 Intravenous cidofovir also may cause uveitis and/or hypotony (low intraocular pressure), which may cause visual loss. A study of long-term follow-up of patients with CMV retinitis treated with cidofovir reported the incidence of cidofovir-related uveitis as 0.20/PY. Regular ophthalmologic monitoring for the development of cidofovir-related ocular side effects is necessary.
Despite the use of systemically administered anti-CMV drugs as maintenance therapy, relapse of CMV retinitis is a nearly universal phenomenon unless there is immune reconstitution. Although reinduction with the same drug may control the retinitis, the interval between the relapses successively declines, suggesting a decreasing ability of single-agent monotherapy to control CMV retinitis over time.1,53 The primary reason for relapse appears to be the limited intraocular penetration of systemically administered anti-CMV drugs.56–58 The efficacy of the ganciclovir implant, which delivers higher levels of ganciclovir to the eye and effectively suppresses retinitis in patients with newly diagnosed CMV until it runs out of drug,45 supports this idea. The Cytomegalovirus Retinitis Retreatment Trial evaluated treatment strategies for patients who had relapsed.59 In this trial, combination intravenous ganciclovir and foscarnet were substantially more effective than either monotherapy with ganciclovir alone or foscarnet alone in patients with relapsed retinitis. Although not more toxic and not associated with any greater impact on general health or mental health, combination therapy did have a greater negative treatment impact on quality of life because it required two intravenous infusions rather than one. In this trial, switching from one monotherapy to another was not more effective than staying on the same monotherapy for the treatment of relapsed retinitis.59 Given the in vitro synergy of foscarnet and ganciclovir, the superiority of combination therapy perhaps is not surprising. Other approaches to combination therapy, including the use of the ganciclovir implant and intravenous foscarnet, oral ganciclovir and intravenous foscarnet, and intravitreous foscarnet combined with another mode of ganciclovir therapy, have been used and appear to have merit for the treatment of frequently relapsing retinitis.
Ganciclovir Implant
The ganciclovir implant contains a reservoir of ganciclovir that is surgically implanted into the eye. The implant slowly releases ganciclovir and obtains sustained intraocular levels of ganciclovir four to five times those achievable with systemically administered drugs. In patients with newly diagnosed retinitis, the implant suppresses retinitis for 6–8 months until it runs out of drug.45,61,62 Scheduled replacement of the implant every 6–7 months appears to be able to effectively suppress the retinitis in patients with newly diagnosed disease and prevent its relapse. The implant appears to be less effective in patients with relapsed disease who have been previously treated with ganciclovir. Approximately 75–85% of relapsed patients will respond.59–61 Because placement of the ganciclovir implant requires an intraocular surgical procedure, it is associated with the typical complications of ocular surgery, including bacterial endophthalmitis, and vitreous hemorrhage. Severe vision-threatening complications occur in less than 5% of patients. Although there were concerns that the implant could increase the retinal detachment rate in patients with CMV retinitis, studies have shown no increase in rate of retinal detachment between eyes with CMV retinitis treated with systemic therapy and those treated with the ganciclovir implant.61–63 The surgical procedure is associated with a transient mild blurring of the patient’s vision in the immediate postoperative period caused by a refractive change while the eye heals. However, the vision typically returns to normal within 1–2 weeks.45 The primary disadvantage of the ganciclovir intraocular device is that it provides no systemic therapy for patients with CMV retinitis. Most patients with CMV retinitis have positive blood and/or urine cultures for CMV.1,33 Prior to HAART, the use of the ganciclovir implant alone was associated with an incidence of contralateral ocular disease of 50% at 6 months and visceral disease of 31%,45 rates which were higher than those seen in patients treated with systemically administered drugs.53 As such, most patients treated with the ganciclovir implant also are given oral ganciclovir or valganciclovir in order to decrease the probability of involvement of the second eye and/or of the viscera. A clinical trial comparing implant with oral ganciclovir to the implant alone and to intravenous ganciclovir showed that the use of oral ganciclovir decreased the incidence of contralateral ocular and visceral disease when compared to the implant alone.62 With the advent of HAART and immune recovery, oral ganciclovir or valganciclovir still is used as adjunctive therapy following placement of a ganciclovir implant in patients who are not taking HAART or have failed HAART and whose CD4+ T-lymphocyte counts remain below 100 cells/μL. In patients taking HAART who have immune recovery, oral ganciclovir or valganciclovir therapy typically is discontinued after 3–6 months of CD+ T cell counts >100–150 cells/μL.
The Ganciclovir Cidofovir Cytomegalovirus Retinitis Trial compared the regimen of the ganciclovir implant with oral ganciclovir to a regimen of intravenous cidofovir. This trial was performed in the HAART era and detected no difference between the two regimens in the rate of relapse of the retinitis.64 In this trial, the rate of progression in the cidofovir group was substantially less than that seen in patients treated with systemic-only therapies in the era before HAART. These data suggest that HAART may have had an effect on retinitis progression, even though it did not have sufficient effect to prevent CMV retinitis.64
An alternative approach to the use of intraocular therapy for the treatment of CMV retinitis has been repetitive intravitreous injections.65–69 Intravitreous ganciclovir typically is given 2 mg two to three times weekly as induction therapy, and once weekly as maintenance therapy.65–67 Intravitreous foscarnet is given 2.4 mg two to three times weekly for 2–3 weeks as induction therapy and once weekly as maintenance therapy.68 Intravitreous cidofovir is associated with uveitis and hypotony, and therefore it is not used in clinical practice.
Fomivirsen
Fomivirsen is a 21-nucleoside phosphorothiolate olignucleotide which hybridizes to complementary mRNA that encodes the proteins of the major immediate-early region (IE2) of CMV.38 The mechanism of action is an antisense mechanism in which the affected mRNA site shuts down the translation and production of specific proteins. Fomivirsen does not cross the blood-ocular barrier, and therefore, is given as an intra-vitreous injection only. The induction dose of fomivirsen is 330 μg in a single dose every 2 weeks for two cycles. The maintenance dose is one 330 μg intravitreal injection once every 4 weeks. Fomivirsen has been shown to be effective in the treatment of CMV retinitis in randomized, controlled, clinical trials.38,70–72 No systemic side effects attributable to fomivirsen have been found in patients receiving the drug.71 Ocular effects have included intraocular inflammation, cataract, and increased intraocular pressure.38,70–73
Management Strategy
Management of the patient with CMV retinitis requires close cooperation between the ophthalmologist and the patient’s primary care provider and/or infectious disease physician. Ophthalmologic examination, including dilated indirect ophthalmoscopy, should be performed at the time of diagnosis of the retinitis and monthly thereafter for patients who remain immunocompromised. For patients who are treated with the ganciclovir implant, typical postoperative follow-up (at 1 day, 1 week, and 1 month after the surgical procedure) also is required. Routine (monthly) fundus photographs using a standardized photographic technique that documents the entire photographic area of the retina provides the optimal method for following patients.40,53 The ophthalmologist can then compare the patient’s current appearance to the previous photographs and detect early relapse of the retinitis. The use of photographs is superior to ophthalmoscopy alone or ophthalmoscopy and retinal drawings.
Resistance
Patients with CMV retinitis treated with long-term maintenance therapy may develop CMV which is resistant to ganciclovir, foscarnet, or cidofovir.74–81 In the pre-HAART era, the rate of developing resistant CMV was ∼0.25/PY for each drug.77,80–82 The mechanism for low-level ganciclovir resistance is a mutation in the CMV UL97 gene. This gene encodes for the phosphotransferase that catalyzes the first phosphorylation step of ganciclovir to ganciclovir triphosphate.83–84 Eighty to ninety percent of all ganciclovir-resistant CMV isolates will have a mutation in the CMV UL97 gene.83,84,87 For high-level ganciclovir resistance, mutations in the CMV UL97 gene and the UL54 gene, the DNA polymerase gene, contribute to ganciclovir resistance.87–90 Mutations in both the UL97 and in the UL54 genes appear to be responsible for cross-resistance to cidofovir as well.87
The detection of a ganciclovir-resistant isolate from either the blood or urine is associated with poorly controlled disease and an increased risk of adverse ocular outcomes.79,80,91 Although a detectable CMV viral load in the blood correlates well with both genotypic and phenotypic resistance92 as well as progression of CMV retinitis, the positive predictive value of CMV viral load for resistance is reported as 8%, making it of limited clinical utility as a marker for viral resistance.93 In the past culture and susceptibility methods for detecting phenotypic antiviral resistance have been labor intensive and not used routinely in clinical practice. However, sequencing of the CMV UL97 gene may be performed on PCR-amplified blood specimens, a procedure that can be performed in <48 h as opposed to >4 weeks for standard cultures, and this more rapid method for the detection of resistant CMV may prove to be clinically useful in the future.94
Retinal Detachments
In the era prior to HAART, retinal detachments were a common complication of CMV retinitis.1,53,95–97 In long-term studies conducted prior to 1995, the incidence of a retinal detachment in either eye of a patient with CMV retinitis was ∼25% at 6 months after diagnosis of retinitis and 50–60% at 1 year.1,53,96 The detachment rate in an eye involved by CMV retinitis was 38% by 1 year,98 and a patient with a CMV retinitis-related retinal detachment in one eye would have a 28–46% chance of developing a detachment in the other eye.1,99 With the advent of HAART, CMV retinitis-related retinal detachment occurs less frequently. In a study of the incidence of retinal detachment in 773 eyes with CMV retinitis from 511 patients with AIDS from both the pre-HAART and HAART eras, patients treated with HAART had a 60% reduction in the retinal detachment rate when compared to patients not taking HAART.63 A multicenter cohort study100 found an overall incidence of CMV retinitis-related retinal detachment of 0.06/PY, which represents an approximate 80% reduction from the incidence of retinal detachment in the pre-HAART era. The rate of retinal detachment reported among patients with CD4+ T-lymphocyte counts <50 cells/μL (0.30/PY) was similar to the rate reported in the pre-HAART era (0.50/PY).100
Prior to HAART, repair of CMV retinitis-related retinal detachments typically consisted of a vitrectomy surgical technique with silicone oil injection, with 75–85% of patients achieving an ambulatory visual acuity in the repaired eye.99,101 Surgical repair without the use of silicone oil was associated with unacceptably high failure rates for retinal reattachment.96 Long-term retinal reattachment repair using silicone oil often is associated with cataract formation, necessitating cataract surgery in these patients. Delimiting laser photocoagulation has been used to check the spread of small peripheral retinal detachments.102 Most, if not all, of these detachments will break through the laser barrier ultimately, but some patients may be spared vitreoretinal surgery, and the approach is useful in some patients. Overall however, because of improved control of CMV retinitis among patients treated with HAART, and because patients with immune recovery due to HAART are more apt to mount an inflammatory response and form scarring allowing for ease of reattachment of the retina, surgical repair without silicone oil as well as laser barrier therapy are being used more often and with greater success, although the published experience remains limited.102–104
Prophylaxis
Two published studies evaluated oral ganciclovir as prophylaxis to prevent the occurrence of CMV disease in patients at high risk for the development of CMV retinitis. Spector et al105 reported that oral ganciclovir prophylaxis resulted in a 49% reduction in the incidence of CMV disease, primarily retinitis. In contrast, Brosgart et al106 used a similar, but not identical study design, and reported that oral ganciclovir was ineffective as primary prophylaxis. Due to the expense of oral ganciclovir as CMV primary prophylaxis,107 the conflicting results of the two studies, and the decreased incidence of CMV disease as a consequence of HAART, primary prophylaxis for CMV disease is not widely used, nor is it recommended in the US Public Health Service (USPHS)/Infectious Disease Society of America (IDSA) guidelines.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree