Site
Procedure
Gastric MALT lymphoma
Stomach
• Endoscopic ultrasound
• H. pylori status (by IHC)a
• FISH or molecular assay for the t(11;18) translocation
Non-gastric MALT lymphoma
Breast
• Mammography and MRI (or CT scan)
Intestine, small
• Endoscopy
• Small bowel series (double contrast X-ray examination of the small intestine)
• Campylobacter Jejuni search in the tumor biopsy by PCR, IHC or in situ hybridization
Intestine, large
• Colonoscopy
Lung
• Bronchoscopy + Broncho-alveolar lavage
Ocular adnexa
• MRI (or CT scan)
• Ophthalmologic examination
• Chlamydophila psittaci in the tumor biopsy and PBMNCs by PCR
Salivary glands
• ENT examination and echography
Skin
• Borrelia Burgdorferi in the tumor biopsy by PCR
Thyroid
• Echography ± CT scan of the neck thyroid function tests
The value of the positron emission tomography (PET) scan is still unclear. In general, the use of PET-CT scan in the routine staging of MZL is not recommended [22, 24], except for selected cases (i.e., when a transformation to high-grade lymphoma is suspected). However, there is some evidence that many non-gastric sites are usually PET-positive [31]. In a meta-analysis of the published literature, the pooled detection rate of 18F-FDG PET or PET/CT in MALT lymphoma was 71 % and appeared particularly high in the pulmonary (94 %) and head and neck (90 %) localizations, showing that this type of lymphoma can often be FDG-avid and suggesting a potential clinical role of PET/CT in the initial evaluation of these patients, especially when the disease is apparently localized and radiotherapy is planned [45].
6 Treatment
6.1 Extranodal MZL of MALT Type
The aforementioned etiologic association with some chronic infections has therapeutic implications in patients with MZL. Indeed, H. pylori eradication with antibiotics can lead to gastric MALT lymphoma regression in 60–100 % of the cases [46–51]. Anti-infectious treatments for non-gastric MZL are, however, largely investigational.
6.2 Gastric Marginal Zone Lymphoma of MALT Type, HP Positive
Figure 1 reports the algorithm of our treatment recommendation for limited and advanced-stage HP-positive gastric MALT lymphoma. Eradication of H. pylori with antibiotics should be the sole initial therapy for localized H. pylori-positive gastric MALT lymphoma, where this treatment can induce lymphoma regression and long-term clinical disease control in most patients [2]. Several effective anti-HP treatments are available. The choice should be based on the epidemiology of the infection and on the expected antibiotic resistance in different countries. The most commonly used regimen comprises a proton pump inhibitor (PPI) associated with clarithromycin and amoxicillin, administered for 10–14 days. Metronidazole can be used instead of amoxicillin for penicillin-allergic patients. Other regimens comprising H2-blockers or bismuth can be also effective. Breath test for H. pylori assessment should be repeated at least six weeks after the eradication therapy and at least two weeks after withdrawal of the PPI. In case of HP-eradication failure, a second-line therapy should be tried with a different triple- or a quadruple-therapy regimen [22] (Table 2). Histological remission is usually achieved within six months from H. pylori eradication. However, the length of time necessary to obtain a lymphoma regression ranges from very few months to more than 12 months. Hence, it is reasonable to wait for at least 12 months before starting another treatment in patients who achieve a clinical and endoscopic remission together with eradication of H. pylori, although histological residual lymphoma persists [2, 52]. The interpretation of the residual lymphoid infiltrate post-treatment in gastric biopsies can be very difficult. Differences in the response criteria adopted in the individual studies may explain the wide range of reported remission rates. Indeed, there are no uniform criteria for the definition of histological remission [2, 52]. Comparison with previous biopsies should be carried out to assess response, and we recommend the Group d’Etude des Lymphomes de l’Adult (GELA) scoring system as a reproducible method [53] (Table 3).
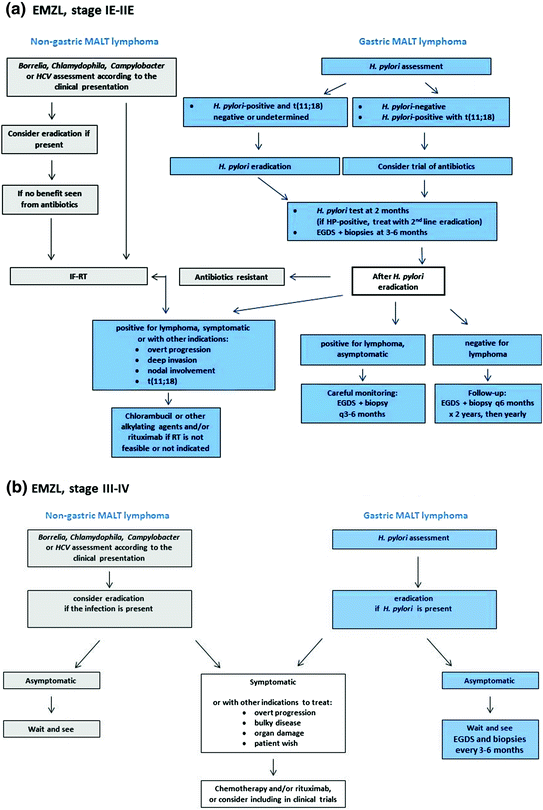

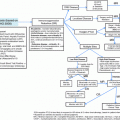
Fig. 1
Algorithm for the treatment of patients with localized (Panel a) or disseminated (Panel b) MALT lymphomas. In principle, to remove an antigenic stimulation that may favor a relapse, eradication of putative driver chronic infections (H. pylori for gastric lymphomas, B. burgdorferi for cutaneous lymphoma, C. psittaci for lymphoma of the ocular adnexa, C. jejuni for IPSID, and HCV for salivary glands or subcutaneous lymphomas) should be given also to patients with disseminated disease together with the required lymphoma treatment. This may reduce the risk of gastric cancer in patients with H. pylori gastritis and liver cancer in those with chronic HCV infection
Table 2
Schedules for H. pylori eradication
Scheme | Drugs | Recommendation |
|---|---|---|
Triple therapy | • PPI (standard dose mg twice daily) | • First line (low prevalence of clarithromycin resistance) |
• Clarithromycin (500 mg twice daily)a | ||
• Amoxicillin (1,000 mg twice daily) for 10–14 days | ||
Triple therapy | • PPI (standard dose mg twice daily) | • First line (low prevalence of clarithromycin resistance) |
• Clarithromycin (500 mg twice daily)a | ||
• metronidazole (400 or 500 mg twice daily)b for 10–14 days | ||
Quadruple therapy | • PPI (standard dose mg twice daily) | • First line (high prevalence of clarithromycin resistance) |
• Metronidazole (500 mg three times a day) | ||
• Tetracycline 500 mg four times a day | ||
• Bismuth sub citrate 120 mg four times a day for 10–14 days | • Second-line treatmentc |
Table 3
GELA criteria for post-treatment histological evaluation of gastric endoscopic biopsies in gastric—MALT lymphoma [53]
Gela grading score | |
|---|---|
CR, complete histological remission | • Normal or empty LP and/or |
• Fibrosis with absent or scattered plasma cells and small lymphoid cells in the LP | |
• No LEL | |
pMRD, probable minimal residual disease | • Normal or empty LP and/or |
• Fibrosis with aggregates of lymphoid cells or lymphoid nodules in the LP/MM | |
• Focal or absent LEL | |
rRD, responding residual disease | • Focal empty LP and/or |
• Fibrosis with dense, diffuse, or nodular lymphoid infiltrate extending around glands in the LP | |
• Focal or absent LEL | |
NC, no change | • Dense, diffuse, or nodular lymphoid infiltrate |
• LEL usually present | |
In fact, histological evaluation of gastric biopsies repeated on a fixed schedule is crucial for the follow-up in order to rule out either the disease persistence or the appearance of early epithelial changes, which may indicate an incoming gastric carcinoma development, especially when H. pylori infection persists. Once H. pylori eradication has been demonstrated (by breath test or by a monoclonal stool antigen test), it is recommended to repeat EGD with multiple biopsies 2–3 months after treatment to exclude lymphoma progression, and subsequently (every 6 months for 2 years) to monitor the histological lymphoma regression [22].
Gastric MALT lymphoma less commonly spreads to distant organs, and the frequency of the histological transformation into diffuse large B-cell lymphoma (DLBCL) is low. Transient histological relapses can be observed in endoscopic biopsies during long-term follow-up, but they tend to be self-limiting, and especially without the stimulus from H. pylori reinfection, they do not implicate a true clinical relapse. Hence, when persistent but not progressive residual disease or histological relapse is documented, a “wait and see” policy seems safe [46, 49, 54, 55]. Nevertheless, a long-term careful endoscopic and systemic follow-up (clinical examination, blood counts and minimal adequate radiological or ultrasound examinations every 12–18 months) is strongly advisable for all patients. Furthermore, the risk of gastric adenocarcinoma among individuals with gastric MALT lymphoma has been reported to be six fold higher, while the risk of other non-Hodgkin lymphomas should be considered higher than in the general population [56, 57].
An obvious prerequisite for a response to antibiotics is the presence of a H. pylori infection. However, there are reports of lymphoma regression following antibiotics also in H. pylori-negative patients, and first-line therapy with antibiotics has to be considered at least in those patients without the t(11;18) translocation, possibly due to a false-negative test or to infection by other Helicobacter species [52]. The response rates of lymphomas are around 70-90 % for the mucosa-confined lymphomas and decrease for the tumors infiltrating the submucosa, the muscularis propria, and the serosa [58–62]. At the same time, the involvement of perigastric lymph nodes, detected by either CT scan or endoscopic ultrasound, rarely respond to antibiotics [60–62]. Moreover, the presence of a high-grade lymphoma component and a history of autoimmune disease were associated with antibiotic therapy resistance [63]. Nearly all gastric lymphomas with t(11;18) translocation will not respond to H. pylori eradication therapy [62, 64]. The t(11;18) is also associated with the resistance to chlorambucil or thalidomide as single agents [65, 66]. Notably, data on a very small series of 13 patients have suggested that the combination of chlorambucil and rituximab is active in t(11;18)-positive cases [67].
H. pylori eradication therapy should be considered in all gastric MALT lymphomas, independent of the stage [22], but patients with symptomatic disseminated disease should also be considered for systemic treatment, e.g., the combination of rituximab and chemotherapy. The role of immunochemotherapy will be further discussed in the following sections which present the management of non-gastric presentations.
6.3 Gastric HP-Negative or Antibiotic-Resistant MALT Lymphoma and Non-gastric MALT Lymphoma
No definite guidelines exist for the management of patients with H. pylori-negative gastric-lymphoma, for patients failing anti-HP treatment, or for the non-gastric localizations. In H. pylori-negative cases, a regression of the lymphoma after antibiotic treatment is unlikely and the immediate start of oncological treatments should be considered. Nevertheless, a course of anti-HP treatment may be considered since occasional lymphoma responses have been reported [2, 52]. An oncological treatment should, eventually, be considered when no signs of lymphoma regression are seen at a repeated endoscopy assessment 2–3 months after antibiotics administration. No significant survival difference between patients who received different initial treatments (including chemotherapy alone, surgery alone, surgery with additional chemotherapy, and radiation therapy) has been shown [68, 69]. Radiotherapy may be the favored choice for patients with localized disease HP-negative or for patients who do not achieve a lymphoma regression following antibiotic therapy [70]. Indeed, involved-field radiotherapy to the stomach and perigastric lymph nodes has results in excellent disease control and most reports support the use of a moderate dose (24–30 Gy given in 3–4 weeks) [71–74].
Radiotherapy has become a standard treatment, at least in North America, also for most non-gastric localized presentations. Literature reports a high rate of local control in MALT lymphoma, with a high proportion of patients likely to be cured[75–84 ]. The use of radiotherapy in this setting has been recommended by the NCCN Clinical Practice Guidelines in Oncology [85]. The modern radiotherapy techniques, such as three-dimensional conformal radiotherapy and intensity modulated radiotherapy, allow an accurate determination of the clinical target volume, thus reducing the toxicity to surrounding organs [73, 86]. The curative doses required (25–35 Gy) are generally associated with mild and reversible acute toxicity and a low risk of long-term side effects, although special caution should be given for specific localizations such as the ocular adnexa or the lung [72, 73, 74, 86].
In patients with disseminated non-gastric MALT lymphoma, observation with a careful monitoring can be often an adequate initial approach. When treatment is required, there is no consensus for the choice of treatment, but rituximab alone or chemotherapy with or without rituximab appear the most appropriate choice. The treatment approach of disseminated MALT lymphomas is the same in patients with primary gastric and non-gastric origin, and the enrollment in controlled clinical trials is advisable. Indeed, there is no standard recommendation, as only a limited number of drugs and regimens have been specifically tested in MALT lymphomas [2]. Oral alkylating agents (either cyclophosphamide or chlorambucil) or purine nucleoside analogues (fludarabine, cladribine) are active as single agents [87, 88]. Rituximab monotherapy has also been tested in phase II studies [89, 90]. However, there is not yet a widely accepted standard immunochemotherapy regimen. Recently, the efficacy and safety of the combination of rituximab plus chlorambucil has been proven in a phase III study of the International Extranodal Lymphoma Study Group (IELSG) in gastric (failing antibiotics) or non-gastric MALT lymphomas. In comparison with either rituximab or chlorambucil given as single agent, chlorambucil plus rituximab resulted in significantly superior complete remission, progression-free and event-free survival rates; however, no overall survival benefit was shown [91, 92]. The combination of rituximab and bendamustine [93] as well as the combination of fludarabine and rituximab has also shown high rates of disease control in smaller non-randomized studies [93]. However, the hematological and infectious toxicity observed with the latter regimen, both during and after therapy, was significant in this patient population [94]. Aggressive anthracycline-containing chemotherapy regimen should be reserved for patients with high tumor burden (bulky masses, unfavorable International Prognostic Index) or for those with histological transformation [95].
6.4 Antibiotic Treatment in Non-gastric MALT Lymphoma
The role of antibiotic therapy in patients with non-gastric MALT lymphoma is unclear [96]. Some anecdotal reports described regressions of non-gastric MALT lymphomas in H. pylori-infected patients after H. pylori eradication [97–99], but this approach is not effective in the majority of patients with non-gastric localization [100]. Antibiotic therapy appears nowadays a reasonable first-line therapy for patients with ocular adnexa MALT lymphoma and may be considered for some cutaneous marginal zone lymphoma; however, it remains experimental in other non-GI localization of MALT lymphomas.
6.5 Antibiotic Therapy for Ocular Adnexa MALT Lymphoma (OAMZL)
The finding that Chlamydophila psittaci has been detected in up to 80 % of Italian patients with OAMZL provided the rationale for the antibiotic treatment of localized lesions [5, 7], and a pivotal Italian experience showed that the eradication of C. psittaci infection using doxycycline for patients with OAMZL may result in lymphoma regression in approximately 50 % of patients, including pre-treated patients and patients with regional lymph node involvement [101, 102]. Following this first demonstration, a prospective international phase II study was later conducted by the IELSG. In this study, C. psittaci DNA was detected in nearly 90 % of the lymphoma biopsy specimens. Thirty-four patients were treated front line with doxycycline and assessed for chlamydia eradication and lymphoma response. Chlamydia eradication was achieved in 14/34 patients (48 %), and six patients obtained a complete lymphoma regression (overall response rate, ORR 65 %). At a median follow-up of 37 months, the 5-year progression-free survival was 55 %. Moreover, in this study, a consistent concordance between C. psittaci detected in tumor tissue and C. psittaci on conjunctival swab indicated that conjunctival swab may be a simple, noninvasive tool for monitoring the infection [6]. Globally, doxycycline has been tested in 120 patients with OAMZL, with an ORR of around 50 % [96]. The median time for response after antibiotic therapy is 6 months. Analogous to H. pylori eradication in gastric MALT lymphoma [52], in some patients, responses are slow and may require up to 36 months [102]. Further investigations are warranted to clarify the appropriate time for starting a different treatment after doxycycline failure, the optimal schedule of doxycycline as well as to identify other potential infective agents, and improve the eradicative antibiotic efficacy [6, 103, 104].
6.6 Antibiotic Therapy for Primary Cutaneous Marginal Zone Lymphoma (PCMZL)
The term “pseudolymphoma” refers to a typical cutaneous B-cell infiltration known to be induced by Borrelia burgdorferi chronic infection. B. burgdorferi has also been reported to have a potential pathogenetic role in marginal zone lymphomas of the skin. Some case reports have shown that the eradication of B. burgdorferi following ceftriaxone therapy resulted in regression of an associated cutaneous marginal zone lymphoma [4]. However, the evidence is based on a limited number of patients [96], and therefore, no recommendations can be made.
6.7 Immunoproliferative Small Intestinal Disease (IPSID)
IPSID has a long natural history, often over many years, including a potentially reversible early phase. If left untreated, however, the lymphoma can undergo a histologic transformation to a DLBCL. In its early phases, IPSID can be treated with prolonged antibiotic therapy (i.e., tetracycline or metronidazole and ampicillin for at least 6 months), which can lead to lymphoma regression. These results may suggest a role for an infectious agent, Campylobacter jejuni seeming the best candidate [8]. Anthracycline-containing regimens, combined with nutritional support plus antibiotics to control diarrhea and malabsorption, represent the best chance of cure for patients with advanced-stage disease. Surgery has no therapeutic role, since the lymphoma usually diffusely involves the intestine.
6.8 Splenic MZL
Most patients with SMZL can initially be managed with a “wait and see” strategy. Cytopenia or symptomatic massive splenomegaly is the main indication for treatment start. When treatment is needed, therapeutic options are splenectomy, chemotherapy, and rituximab alone or in combination with chemotherapy [24].
Splenectomy has been for a long time the therapy of choice. It allows prolonged remissions with rapid alleviation of splenomegaly-related symptoms, accompanied by resolution of cytopenia and disappearance of circulating lymphocytes in around 90 % of the cases. Even when bone marrow involvement and lymphocytosis persist, the time to next treatment may be longer than 5 years [32]. However, SMZL is a systemic disease, and splenectomy cannot be considered a curative option. Moreover, it is a major surgical procedure with significant morbidity and non-negligible mortality, especially in older patients [105].
There is no evidence for a survival benefit when chemotherapy is added to splenectomy. Chemotherapy alone has been used for patients unsuitable for splenectomy or relapsing after surgery. Alkylating agents (i.e., chlorambucil or cyclophosphamide) alone or in combination (i.e., CHOP) with fludarabine monotherapy have shown to be effective. More recently, since most patients are elderly and at high risk for surgery, frontline immunotherapy with rituximab alone or in combination with chemotherapy has become more and more widely used [105–109]. In a Greek study, 58 patients were treated with rituximab at a dose of 375 mg/m2 per week for 6 weeks as induction, followed by rituximab maintenance in 43 patients (375 mg/m2 every 2 months for 1–2 years). The overall response rate in this study was 95 %, with almost half of responses being complete, while the 5-year progression-free survival was 77 %. Maintenance therapy with rituximab appeared to induce a significantly longer response duration (the 5-year progression-free survival was 84 % for patients receiving maintenance and 36 % for patients without maintenance) [108].
A survey on the survival outcomes after surgery or rituximab-based systemic therapy in the Surveillance Epidemiology and End Results-Medicare database (SEER) included 227 patients, diagnosed between 2000 and 2007, and treated with splenectomy (68 %), rituximab alone (23 %) or in combination with chemotherapy (9 %) within 2 years from diagnosis. It showed higher rates of hospitalizations, infections, transfusions, and cardiovascular or thromboembolic events after chemoimmunotherapy than after splenectomy. Conversely, there was no significant difference in the complication rates between groups treated with splenectomy or rituximab alone [109].
Other retrospective series of rituximab monotherapy, including both chemotherapy-naive and refractory patients, showed overall responses of 88–100 % with marked and prompt regression of splenomegaly and improvement of cytopenias with overall survival comparable to that reported after splenectomy. Sustained responses occurred both with and without rituximab maintenance and relapsed patients responded to second courses of rituximab monotherapy [106]. The remission rates and durations after rituximab, given either alone or with chemotherapy, were significantly better than after chemotherapy without rituximab in the same patients, with manageable toxicity [107]. The combination of rituximab with chemotherapy may further improve progression-free survival, but further evaluation with confirmatory prospective trials is warranted [105–107]. The available evidence, despite being mainly based on retrospective studies, seems to suggest that rituximab could replace splenectomy as first-line treatment, particularly in SMZL patients over the age of 65 years. In these patients, the risk of lymphoma-related death and overall survival were similar to rituximab or splenectomy as initial therapy, indicating that single-agent rituximab may carry the most favorable risk/benefit ratio in this population [109], but optimal schedule and long-term outcome have not been fully defined yet.
For patients with SMZL HCV-associated, there is evidence that antiviral treatment may be an acceptable option. As already mentioned, the use of antiviral therapy to treat HCV-associated lymphomas was established after the demonstration by Hermine et al. [9] of splenic marginal zone lymphoma regression following HCV eradication. Later, other reports confirmed that antiviral therapy can be an efficacious frontline therapy for HCV-associated SMZL and nodal MZL [10, 12, 110, 111]. HCV-RNA clearance is needed to attain lymphoma response [111], and the achievement of a virologic response can be followed by a lymphoma regression in up to 75 % of the cases. At present, in HCV-positive patients with marginal zone lymphoma who do not need immediate conventional treatment for lymphoma, antiviral treatment with pegylated-interferon-α and ribavirin should be considered as first-line treatment [24, 111]. However, novel and better tolerated antiviral agents are under development and will hopefully make easier the treatment of both lymphoma and hepatitis. Indeed, less toxic and shorter interferon-free regimens will very soon become a suitable option, and this may be particularly advantageous for the treatment of lymphoma patients with interferon-resistant HCV genotypes [112, 113].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree