WHO (1999)
ATP-III (2001)
IDF (2005)
Fasting glucose
Diabetes mellitus, impaired glucose tolerance
>110 mg/dl
>100 mg/dl or previously type-II diabetes
Blood pressure
≥140/90 mmHg or use of medication
>130/85 mmHg or use of medication
>130/85 mmHg or use of medication
Dyslipidemia (TG)
≥150 mg/dl
≥150 mg/dl
≥150 mg/dl
Dyslipidemia (HDL-CH)
<35 mg/dl for males
<40 mg/dl for males
<40 mg/dl for males
<39 mg/dl for females
<50 mg/dl for females
<50 mg/dl for females
Central obesity
WHR > 0.9 in males
Waist circumference
Waist circumference
>0.85 for females and/or BMI >30
≥102 cm for males
>94 cm for males
≥88 cm for females
>80 cm for females of Caucasian race
Microalbuminuria
Urinary albumin excretion ratio ≥20 μg/min or albumin:creatinine ratio ≥30 mg/g
Diagnostic criteria
MD type II or IGT and two criteria
Three or more criteria
Central obesity and two criteria
The metabolic syndrome represents a whole heterogeneous disorder that correlates with high mortality and morbidity rates and high economic and social costs. The syndrome affects 20–25 % of the general population with an increased prevalence with aging, in particular among older people 50–60 years of age. The characteristics of the syndrome are visceral obesity, abnormal lipid and glucose metabolisms and high blood pressure: the specific pathophysiological feature is represented by insulin resistance, which may explain each of the metabolic impairments: the insulin resistance promotes the storage of fat tissue at a visceral level, less sensitive to insulin action, and as a consequence a higher lipolytic function may increase the release of non-esterified fatty acids (NEFAs) into the liver circulation. These NEFAs cause abnormal synthesis of glucose and triglycerides that in turn compromises hepatic insulin clearance. Furthermore, the fat mass is not considered to be simply an energetic store but an active endocrine tissue that releases a large number of adipocytokines, some of which have pro-inflammatory and pro-atherogenic functions (TNF α, IL-6, leptin, adiponectin, etc.) with an active role in modulating insulin sensitivity: for example, the reduced secretion of adiponectin plays a crucial role in inducing insulin resistance, but also in determining the clustering of elevated triglycerides and small, dense LDL particles. Increased leptin secretion may be responsible for sympathetic nervous system overactivity and hypertension, while reduced omentin may play an important role in the development of atherogenic processes [5].
Insulin resistance promotes sodium retention at the kidney level, with a consequently negative impact on blood pressure homeostasis. Finally, when the pancreatic compensatory mechanisms for insulin resistance become inadequate, the fasting glucose increases and, because of the reduced glucose cell intake, diabetes progresses: the metabolic syndrome is taking place (Fig. 16.1).
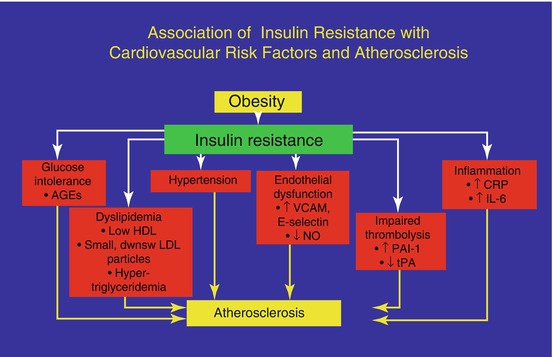

Fig. 16.1
The metabolic impact of the combination of obesity and insulin resistance and cardiovascular risks
Insulin resistance, in turn, may be dependent on different problems: an intrinsic/structural cell disorder, owing to receptoral or post-receptoral function defects, or an acquired disorder, such as excessive weight (overweight or obesity), which increases the amount of fat and induces a change in the receptor binding ability of hormones. Insulin resistance is also dependent on familial predisposition, especially when there are diabetic relatives and often with the combination of many of the elements described above.
16.2 Relationship Among Obesity, MS and Menopausal Transition
As we know, menopausal transition is characterized by an early (approximately 10 years before the start of the menopause) and a progressive increase in FSH levels, linked to a decrease in inhibin production by the ovaries; at the same time, the higher frequency of anovulatory cycles induces a decrease in progesterone during the luteal phase, resulting in relative hyperestrogenism, followed by a long period of hypoestrogenism when the menopause has actually taken place.
At present, although a large number of publications have been produced, the change in body weight during menopausal transition represents a important topic of discussion.
Usually, the modifications of weight are dependent on an increase in energetic impute and/or a decrease in energy consumption, mostly because of physical activity and resting energy expenditure. It is well known that women after the age of 45–50 years, in the absence of changes in their lifestyle (feeding and physical activity), show a progressive increase in bodyweight. What is the cause of this?
Whereas weight gain per se cannot be attributed to the menopause transition, the change in the hormonal milieu at menopause is associated with an increase in total body fat, and in particular there is an increase in abdominal fat [6]. Lovejoy et al. [7] showed that progressively, during perimenopause, there is an increase in abdominal fat mass, concomitant with climacteric hypoestrogenism, with a slow increase in weight despite the reduced trend in total calorie intake (Fig. 16.2).
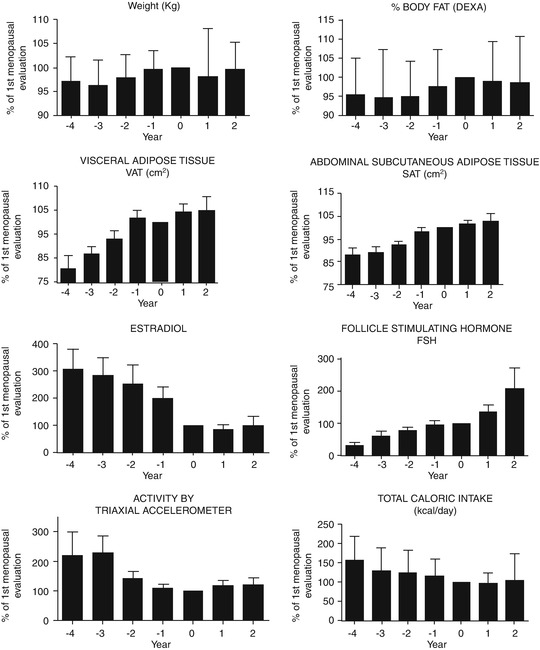

Fig. 16.2
Despite weight that remains the same, there is an increase in body fat mass and in visceral adipose tissue (Modified from Lovejoy et al. [7])
From the clinical point of view, it is important to know the metabolic condition of our patients: during reproductive life, they may have suffered from some metabolic disorders, including thyroid dysfunction or mellitus diabetes and/or insulin resistance, as in the case of polycystic ovary syndrome (PCOS) or a history of being overweight/having obesity. The exposure to environmental factors, until the beginning of childhood, can affect the normal weight and worsens it: in general, personal health history affects the metabolic change that occurs during menopausal transition.
The gonadal steroid hormones have specific metabolic effects during perimenopausal transition. As mentioned before, menopausal transition is characterized by alternating hypoestrogenic and/or hyperestrogenic periods with low progesterone levels. Indeed, during the luteal phase of the menstrual cycle, the typical increase in P/E2 ratio induces an increase in body temperature of about 0.4 °C [8]: this temperature rise causes an elevation of basal metabolism of about 200 kJ (50 kcal) per day [9]. On the contrary, the absence of a progesterone increase during menopausal transition determines the lack of energy consumption under resting conditions typical of the luteal phase (about 50 kcal a day, for 12–14 days: approximately 600–700 kcal every month). This event is at the basis of the increase in fat mass deposition during the perimenopause [10] and does not induce the physiological burning of calories during the luteal phase.
This decline in energy consumption seems to be not exclusively dependent on progesterone deficiency but also on other factors, such as the amount of lean mass, the activity of the sympathetic nervous system (SNS), the endocrine status and the ageing-mediated physiological changes. In fact, the decline in basal metabolism observed in post-menopausal women may also depend on aging [11]. However, the basal metabolism decreases more during menopausal transition than could be attributed to the aging process [12]. Oestrogen depletion probably contributes to the acceleration of this decline. As the perimenopause becomes the menopause, the progressive reduction of oestrogen levels induces a progressive worsening of the insulin resistance, which is also increased owing to the concomitant cortisol increase (typical of aging and the menopause). This latter event induces gluconeogenesis and further promotes insulin resistance. The hypoestrogenism also partly induces a fall in GH levels, which produces an increase in the storage of abdominal fat mass with a decrease in lipid metabolism [13, 14].
Further confirmation of the role of oestrogen in fat mass control comes from experimental studies on animal models where in ovariectomized mice, the oestrogen treatment reduces the fat mass and the adipocyte cell size, independently of the energy intake. In particular, oestradiol seems to accelerate the fat oxidation pathways in the muscles and adipocyte lipolysis [15].
In summary, the decrease in basal metabolism induces the gain in fat mass which, in turn, may contribute to improving the incidence of obesity-related diseases, such as worsening of the cardiovascular profile and type II diabetes. The increase in visceral adiposity further worsens insulin sensitivity, in particular at the liver level, so that higher amounts of insulin are needed to control glucose intake at tissue levels. All these changes determine the increase in the rate of incidence of the metabolic syndrome in overweight/obese women during the menopausal transition.
Recently, it has been suggested that another possible link between menopausal hypoestrogenism and appetite control/weight gain is the increase in levels of orexin-A plasma [16]. This recently discovered hypothalamic neuropeptide is involved in the regulation of feeding behavior, of the sleep–wake rhythm and of neuroendocrine homeostasis [17, 18]. In post-menopausal women, while estrogens are at low levels, plasma orexin-A levels are significantly higher, being part of the induction for some cardiovascular risk factors, such as blood glucose, lipid profile, blood pressure and body mass index [19].
16.3 The Approach to Obesity and Metabolic Syndrome During Perimenopause
The approach to overweight/obese pre-menopausal women has to consider and evaluate a careful anamnesis and medical investigation, especially with regard to the age at obesity onset, any recent relevant weight change and if there are any members of the family with diabetes or other endocrinological diseases and/or obesity The purpose of this check is to exclude any clinical cause that might require specific therapeutic approaches, such as any uncommon secondary obesities, due to genetics, neurological or psychiatric conditions.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree