Age-related Changes in Body Composition
At different ages, the same level of body mass index (BMI), calculated as kg m−2, corresponds to different amounts of fat and fat-free mass. With age, whole-body fat mass (FM) remains relatively stable but increases as a percentage of body weight due to decreased lean mass, whereas both percentage and absolute abdominal fat mass increase. Fat mass peaks at age 60–75 years, whereas muscle mass and strength start to decrease at age 30 years, with accelerated loss occurring after ∼60 years of age.1 In addition, there tends to be a redistribution of fat viscerally and inter- and intra-myocellular fat increases. A number of factors are associated with the age-related changes in body composition. There is a decrease in overall energy expenditure and a significant component of this change relates to decreased physical activity. The decrease in skeletal muscle mass is a consequence of reduced physical activity, increases in pro-inflammatory cytokines (e.g. interleukin-6 and tumour necrosis factor-α) and decreased production or resistance to the actions of anabolic hormones.1 Visceral obesity, which results in an increase in inflammatory cytokines and a decrease in anabolic hormone levels, is associated with insulin resistance. Intramuscular fat infiltration is associated with both increased insulin resistance and accelerated loss of skeletal muscle strength.2 Reduced growth hormone and testosterone levels in obese older people have been associated with decreased muscle mass and strength.
Energy Expenditure
Age-related decreases in energy expenditure are primarily due to decreased physical activity and reduction in resting metabolic rate (RMR). The decline in RMR with age is not entirely attributable to the reduction in fat-free mass (FFM), as RMR in people aged 60 years and above is lower than that of younger individuals after adjusting for FFM, fat mass and gender. In an 8 year longitudinal study of men and women >60 years of age in Germany, the reduction in RMR per decade was 5% in men and 3% in women after adjusting for body composition, and physical activity energy expenditure also decreased, resulting in a reduction in total energy expenditure per decade of 7.5% in men and 6% in women.3 In the Baltimore Longitudinal Study of Aging, RMR decreased with age in both men and women independently of BMI, and the rate of decline was faster at age 70–80 than at age 40–50 years.4
The thermic effect of food (TEF), which is the increase in energy expenditure in response to macronutrient intake and accounts for up to 10% of total energy expenditure, declines with ageing. The TEF after a glucose load has been found to be ∼50% less in healthy men aged 54–75 years than in men aged 19–36 years.5 Furthermore, TEF was higher in men who habitually exercised than their sedentary counterparts in both age groups.5 This suggests an additional mechanism by which regular physical activity may prevent obesity in the elderly. Although obese elderly people have lower basal fat oxidation (adjusted for FFM) than younger individuals, 24 h fat oxidation during exercise is higher in normally sedentary older (65 years) men than younger (25 years) men after adjusting for FFM,6 suggesting that the reduction in nutrient utilization in the obese elderly is largely due to an increase in visceral and intramuscular fat.
Energy Intake
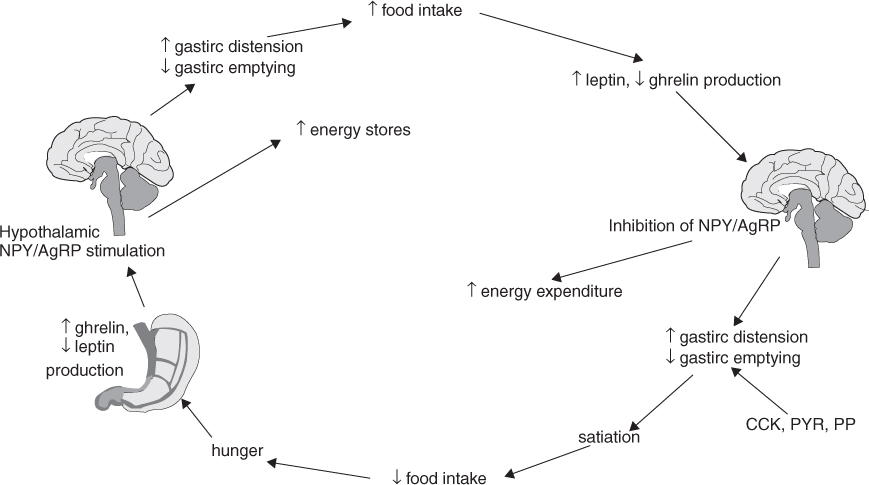
Food intake is regulated by a complex system involving orexigenic (appetite-stimulating) and anorexigenic (appetite-inhibiting) hormones that link hypothalamic satiety centres with gastrointestinal function and energy stores (Figure 20.1). Ghrelin, produced in and secreted from the gastric mucosa, is increased by fasting and low-protein diets and inhibited by somatostatin, growth hormone and a high-fat diet. Ghrelin is the main orexigenic signal that stimulates neuropeptide Y (NPY) and agouti related peptide (AgRP) in the hypothalamus. NPY and AgRP, which are capable of orexigenic stimuli themselves while simultaneously antagonizing anorexigenic melanocortin pathways, increase appetite, gastric motility and triglyceride stores in adipocytes, but reduce energy expenditure, resulting in net body weight gain. Conversely, the actions of NPY and AgRP are inhibited by the anorexigenic stimulus of leptin, secreted from adipocytes, especially after a meal. Obesity is associated with elevated plasma leptin levels in the elderly, as in younger people.7 Other gastrointestinal peptides, such as cholecystokinin (CCK), glucagon-like peptide-1 (GLP-1), pancreatic polypeptide (PP) and peptide YY (PYY) 3–36, act in synergy with leptin to reduce gastric emptying, resulting in increased gastric distension, which promotes satiety.
There is evidence of altered synthesis and/or secretion and reduced responsiveness to centrally secreted and gut hormones regulating appetite and metabolism in elderly people as compared with young adults; this may contribute to age-related changes in appetite and energy regulation. Compared with younger people, the plasma level of the hormone CCK, which is secreted from the proximal intestine in response to ingestion of fat and protein and induces satiation, is higher in the elderly,8 but CCK has a weaker than expected effect in limiting meal size in older people.8 In elderly subjects (mean age 80.7 years), the effect of a carbohydrate-rich test meal to suppress active and total ghrelin levels is limited as compared with younger subjects.9 Elderly people tend to be less sensitive to satiety cues, follow eating patterns according to habit or schedule and under-report energy intake.10
Assessment of Obesity with Increasing Age
Body mass index is of limited utility in assessing obesity in older people due to the age-related changes in body composition. The relationship between BMI and cardiovascular risk and mortality in the elderly is flattened and a high BMI carries a lower risk than in younger people. In contrast, a low BMI, which reflects a low lean body mass, confers a higher risk in older people in comparison with younger people. The combination of a low lean body mass and increased fat mass, known as sarcopenic obesity, confers the highest risk of frailty and death. Sarcopenic obesity increases in prevalence with age and is associated with functional decline, and also other causes of morbidity and mortality in the elderly. Various definitions of sarcopenic obesity have been proposed, such as relative skeletal muscle index (muscle mass divided by height squared) less than two standard deviations below the gender-specific mean of young adults and percentage body fat greater than median (27% in men and 38% in women); or muscle mass in the lower two and fat mass in the upper two quintiles of the population.1 Depending on the definition used and populations studied, the prevalence varies from 4.4 to 9.6% in men and from 3.0 to 12.4% in women.1 Identification of sarcopenic obesity requires precise, simultaneous methods of measuring body fat and muscle mass, such as dual-energy X-ray absorptiometry (DEXA). Bioelectrical impedance analysis (BIA) tends to underestimate fat mass in obese subjects11 and the reference ranges for the definition of sarcopenic obesity have not been widely validated. Computed tomography (CT) scanning is of greater precision, but is relatively expensive and may be less accessible.
Waist circumference (WC) correlates with abdominal fat mass as measured by CT and has been proposed as an additional measure for the definition of obesity in the elderly.7 Adults aged 51–72 years with BMI in the normal range (18.5–25 kg m−2) but WC ≥102 cm (men) or ≥88 cm (women) have a 20% higher risk of all-cause mortality than their counterparts with similar BMI but normal WC.12 Waist:hip ratio (WHR) is an alternative measure that provides some assessment of lean body mass and subcutaneous fat mass. A WHR exceeding 1.0 is associated with increased risk of obesity-related comorbidities, particularly cardiovascular disease. WHR is a better predictor of obesity-associated morbidity and mortality than BMI in the elderly.12–14
Prevalence of Obesity in Older People
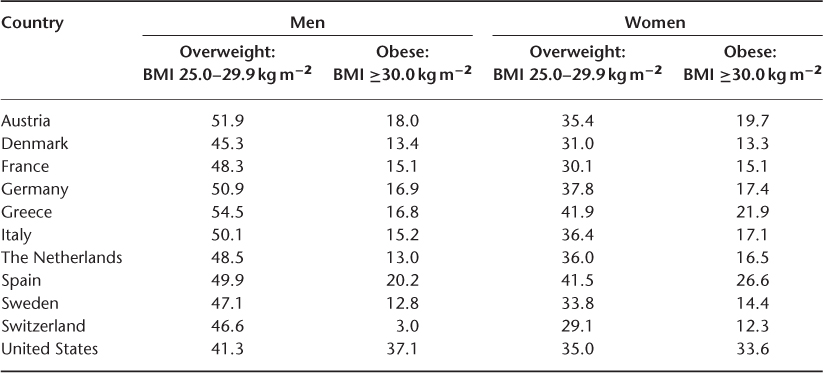
The prevalence of obesity (BMI ≥30 kg m−2) in older people has been increasing in the last decade and ranges from ∼15–40%, with a slightly higher prevalence in women (Table 20.1). In the United States, the prevalence of obesity (BMI ≥30 kg m−2) in men aged 60 years and above increased significantly from 31.8% in 1999 to 37.1% in 2008 and remained similar in women in the same age group at ∼35%.15 The 2004 Survey of Health, Ageing and Retirement found that the prevalence of obesity in adults aged above 50 in 10 European countries ranged from 12.8 to 20.2% in men and from 12.3 to 25.6% in women.16 Obesity will continue to be a major problem in ageing populations worldwide.
Table 20.1 Prevalence (%) of overweight and obesity in older persons.15, 16.
Consequences of Obesity in the Elderly
Overall Mortality
In contrast to young and middle-aged populations, in which the mortality risk increases with BMI, the relationship between BMI and mortality in the elderly may be U-shaped or reverse J-shaped, with increased mortality risk at BMI exceeding 35 kg m−2. The range of BMI which is associated with the lowest risk of mortality in older adults in most retrospective studies ranged from 27 to 30 kg m−2 7, 16 and is 2–5 kg m−2 higher in women than men.7 Several explanations have been proposed for this apparent paradox of overweight elderly having a lower risk of death: overweight people who are more susceptible to the adverse effects of obesity tend to die younger, leaving their peers of similar weight to be included in studies of older subjects; the effects of confounding conditions such as smoking and weight change due to undiagnosed chronic disease are difficult to separate from the effects of obesity per se, and older people may succumb to apparently-unrelated comorbidities. The elderly at greatest risk are those who are simultaneously sarcopenic and abdominally obese.
Body composition, in particular increased abdominal adiposity and reduced muscle mass, as measured by large waist circumference and waist:hip ratio, may be a better predictor of cardiovascular and all-cause mortality than high BMI. In the Rotterdam Study, WC ≥102 cm in men and ≥88 cm in women with normal BMI was associated with 20% higher mortality risk compared with subjects with a combination of normal BMI (18.5–<25 kg m−2) and normal waist circumference12 In a large study of community-dwelling adults aged 75 years and older, increasing WHR was associated with mortality from cardiovascular disease, with WHR exceeding 0.99 conferring the highest risk of mortality in non-smoking men, and WHR >0.90 in non-smoking women was associated with the greatest risk of death.13 In the MacArthur Successful Aging Study, a longitudinal study of high-functioning men and women aged 70–79 years, there was a graded relationship between WHR and all-cause mortality in women (relative risk 1.28 per 0.1 increase in WHR) and a threshold relationship in men (relative risk 1.75 for WHR >1.0 compared with WHR ≤1.0.14
Mobility-related Disability, Functioning and Quality of Life
Aging is associated with reduced muscle strength and mass and increased prevalence of osteoarthritis (OA), which adversely affects mobility, ability to perform activities of daily living and quality of life. Obesity is associated with increased risk of knee OA, possibly due to mechanical strain on weight-bearing joints, although metabolic syndrome is now recognized to be a risk factor for OA. In a community-based study of people aged 65–80 years, up to 96% of obese (BMI ≥30 kg m−2) subjects were frail, as determined by physical performance test scores, peak oxygen consumption and self-reported ability to perform activities of daily living.17 The same study also found that obesity was associated with a lower health-related quality of life, as assessed by the SF-36 physical function scoring. Sarcopenic obesity in particular is associated with high risk of impaired mobility and reduced functioning. The combination of obesity and low muscle strength was associated with the development of mobility-related disability in 930 people aged 65 years and above in the InCHIANTI study in Italy.18 Despite the positive association between weight and bone mineral density, a recent study found that high body weight or BMI increased the risk of vertebral fractures in postmenopausal osteoporotic women.19 Vitamin D deficiency, which is more prevalent in obese sedentary elderly because of reduced exposure to sunlight, impairment of vitamin D synthesis in the skin with ageing and obesity-associated reduction in bioavailability of cutaneous and nutritional vitamin D, is likely to contribute to osteopenia, fractures, muscle weakness and reduced mobility.
Metabolic Syndrome and Type 2 Diabetes Mellitus (T2DM)
The prevalence of the metabolic syndrome (defined by the presence of at least three of the following characteristics: WC >102 cm in men and 88 cm in women, high-density lipoprotein cholesterol <1 mmol l−1 in men and 1.3 mmol l−1 in women, blood pressure >130/85 mmHg, serum glucose >6.1 mmol l−1) increases with age. Increased body fatness and increased abdominal obesity, rather than ageing per se, are thought to be directly linked to the greatly increased incidence of metabolic syndrome and T2DM among the elderly. As in younger people, increased abdominal fat mass is independently associated with the development of metabolic syndrome in older men and women and waist circumference, a surrogate of abdominal adiposity, is positively correlated with dyslipidaemia, fasting glucose and hypertension, independently of BMI and age. Visceral adiposity-mediated inflammation is involved in the pathogenesis of insulin resistance in elderly obese adults. The age-related reduction in insulin sensitivity is likely to be due to increase in adiposity rather than being a consequence of ageing per se.
Fatty Liver
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree