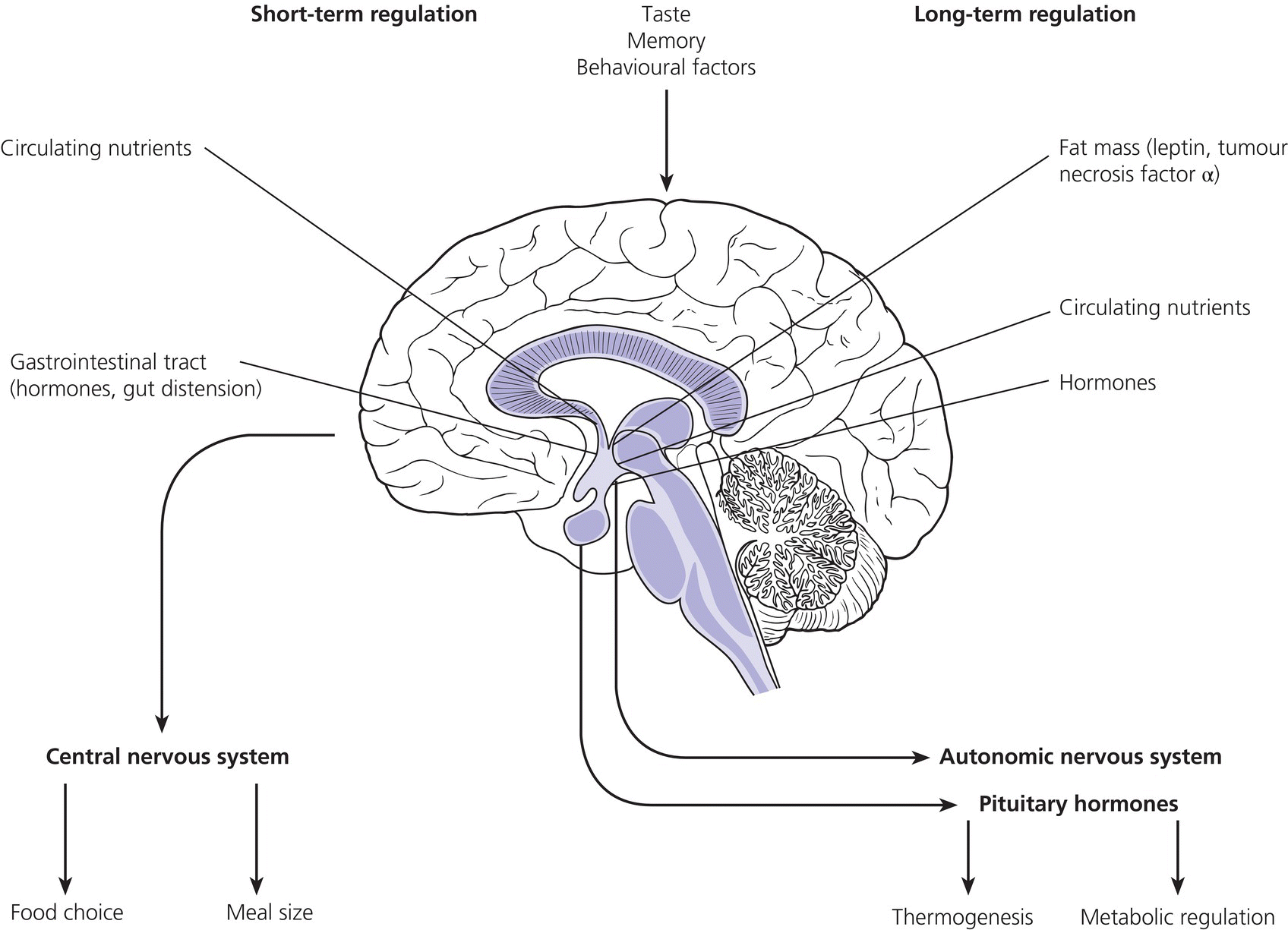
11 In childhood, obesity may be assessed in different ways. Measurement of weight alone is inadequate, given the influence of height on weight. Although obesity may be clinically obvious if the child’s weight centile is greater than the height centile, the severity of obesity is better defined by the use of the body mass index (BMI) in which It should be noted that although excess BMI is good at identifying excess weight, it does not distinguish between fat and lean tissue and in unusual circumstances (e.g. heavy‐weight sports) may not indicate excess fat. Alternatively, body fatness can be assessed from direct measurements of subcutaneous skinfold thickness using skinfold thickness callipers or from measurement of waist circumference. Given the normal variations of BMI, skinfold thickness and waist circumference through childhood, reference to centile charts is required for interpretation of the data. The utility of skinfold thickness and waist circumference measurements is limited by the practical difficulties of obtaining these measurements accurately and so measurement of BMI is currently the best clinical method for identifying and monitoring obesity in childhood. There are no universally agreed definitions of obesity in childhood as there are few data correlating specific definitions with future health risks. In practice in the United Kingdom, children with a BMI above the age‐ and sex‐specific 91st centile can be defined as overweight and those above the 98th centile as clinically obese (see Appendix 1 for sex‐specific BMI centile reference charts). Throughout the industrialized world, there is clear evidence of a rapid increase in the prevalence of obesity in children and adults, with older children more affected than infants and those from Hispanic and non‐Hispanic black communities in the United States more affected than non‐Hispanic whites. In the United States, more than 20% of 12–19‐year‐old children have a BMI that exceeds the 95th centile (the US definition of obesity). An increased risk of obesity is associated with genetic, environmental (Table 11.1), and pathological factors (Table 11.2). It is likely that a combination of an increasingly sedentary lifestyle combined with an excessive calorie intake for need is principally responsible for the rapid increase in the prevalence of obesity noted in recent decades. Table 11.1 Non‐pathological risk factors for obesity. Table 11.2 Pathological causes of excess body weight. Obese children under three years of age without obese parents are at low risk of obesity in adulthood. However, in older children, the presence of obesity becomes an increasingly important predictor of adult obesity, regardless of whether the parents are obese or not, with more than two‐thirds of children who are obese aged 10 years or older becoming obese adults. Parental obesity more than doubles the risk of adult obesity in young children. Twin studies have suggested a heritability of fat mass of 40–70%. The normal amounts of body fat change through childhood, as can be seen from the BMI centile charts (see Appendix 1). Infants gain fat, as a consequence of increasing adipose cell size, relatively rapidly until the age of one year, but then slim down until the age of approximately six years as adipose cells reduce in size. From this point onwards, there is a steady increase in body fat into young adult life (the so‐called ‘adiposity rebound’) associated with increases in adipose cell numbers. There is little difference in the amount of body fat in infant girls and boys. However, after infancy, subcutaneous fat increases more rapidly in girls, particularly during puberty when males demonstrate greater centralization of body fat stores. Obesity occurs when energy intake chronically exceeds energy expenditure. In obese children there are wide variations in energy intake and not all obese children eat excessively. The high calorie density and fat content of the modern diet are associated with an increased risk of obesity. The hypothalamus has a central role in the regulation of energy balance, integrating neuronal, hormonal, and nutrient messages from within the body and transmitting signals which lead to the sensations of hunger or satiety (Figure 11.1). The hypothalamus also influences energy expenditure via autonomic nerve function and the regulation of pituitary hormone release. Many hypothalamic neurotransmitters have been shown to influence energy intake (Table 11.3). Figure 11.1 Influences on the hypothalamic control of satiety. Table 11.3 Factors which influence the central nervous system regulation of energy intake. Energy expenditure consists of the following three components: As a consequence of these influences on energy expenditure, in most obese children, absolute total energy expenditure is increased. However, there is evidence that a low relative metabolic rate (i.e. adjusted for body size) predisposes to weight gain. Complex interactions exist between signalling pathways that control energy intake and satiety and those that regulate fat mass. Adipose tissue may influence the hypothalamic regulation of the energy balance through the secretion of leptin, a hormone which crosses the blood–brain barrier and suppresses the production of neuropeptide Y within the hypothalamus, leading to reduced appetite and increased energy expenditure. However, most obese children have increased leptin concentrations in proportion to their increased body fat mass. Disorders of leptin synthesis or action (leptin receptor mutations) are very rare and account for few cases of obesity. Most children with obesity do not have an underlying pathological cause and have so‐called ‘simple obesity’, also known as exogenous obesity. Such children usually demonstrate rapid growth and physical development. By contrast, children who are obese, short, and growing slowly are much more likely to have an endocrine abnormality or, if associated with dysmorphic features and intellectual impairment, a syndromic cause to their obesity. A careful history and examination are required to distinguish the few children with significant underlying pathology causing their obesity from those who have ‘simple obesity’. The history should include the following details: Figure 11.2 Prader–Willi syndrome. Source: M. Donaldson, Glasgow. On examination, the child should undergo an accurate assessment of height and weight with measurements plotted on height, weight, and BMI centile charts. Height measurements should be compared with the target height range derived from the mid‐parental height centile, as those who are relatively short for their genetic background are more likely to have a syndromic or endocrine cause for their obesity, whereas those who are relatively tall are more likely to suffer from simple obesity. Further assessment of the severity of the obesity can be made from the measurement of waist circumference or triceps and subscapular skinfold thickness, although these measurement techniques require training and are difficult to perform in very obese individuals. Not all pathological causes of obesity will be self‐evident and careful clinical examination is required to identify signs suggestive of certain disorders or complications of obesity. It is particularly important to note the presence of acanthosis nigricans most commonly seen in the axillae or neck (see Figure 1.9), as this may indicate evolving insulin resistance and an increased risk of developing type 2 diabetes. Key signs to note on clinical examination are shown in Table 11.4. The clinical features of syndromes, which may present with obesity, are summarized in Table 11.5. Table 11.4 Clinical signs to look for on examination. Figure 11.3 Polydactyly in a child with Laurence–Moon–Biedl syndrome. Figure 11.4 Shortening of the fourth metacarpal (a) and metatarsal (b) in pseudohypoparathyroidism. Table 11.5 Clinical features of common syndromic causes of obesity (Figures 11.2–11.4). The main adverse effects of obesity are summarized in Table 11.6. In childhood, acute medical complications of simple obesity are usually few and minor. The combination of insulin resistance, hypertension, hypertriglyceridaemia, and a low concentration of high‐density lipoprotein (HDL) cholesterol (so‐called ‘metabolic syndrome’) used to be rarely seen in childhood, although in the United States, nowadays, one in four overweight children in the age group 6–12 years has impaired glucose tolerance and 60% of these have at least one risk factor for heart disease. There are particular concerns about the increased prevalence of obesity and its metabolic complications in ethnic minority groups in both Europe and North America. Furthermore, being overweight in childhood has been shown to be a major risk factor for the development of the metabolic syndrome and coronary artery disease in adult life. Long‐standing obesity leads to children being tall for their age, relative to genetic height potential, and to an advanced bone age (so that adult height is not increased). Why excess body fat has this effect is unclear. Obese children may also have an impaired self‐image and for boys this may be complicated by a marked suprapubic fat pad leading to the penis being buried in fat and, as a result, appearing very small. Table 11.6 Complications of simple obesity. The following investigations should be considered in those in whom there is clinical concern regarding an underlying pathological cause or a possible metabolic complication of obesity: Young infants frequently appear fat and a spontaneous reduction in body fat occurs as part of the normal changes in body composition with increasing age. In general, therefore, it is rare that a slimming regimen needs to be considered in the young child. However, investigations to exclude a pathological cause of obesity should be undertaken in all infants with severe, early onset obesity. If calorie restriction is deemed necessary in infancy, the aim should be maintenance of body weight rather than weight loss, as the latter may lead to specific dietary (e.g. vitamin) deficiencies or growth failure. For many obese children, weight loss down to an ‘ideal body weight for height’ is probably unrealistic. Nevertheless, more modest weight reduction, or even prevention of further weight gain, may produce significant long‐term health benefits. Such goals, which are more likely to be achievable, should be considered when planning individual therapeutic regimens. Unfortunately, there is almost no published evidence for any effective treatment for childhood obesity. Older children with simple obesity should be encouraged to try and control their weight gain by: Once a child has developed significant obesity, both weight loss and long‐term maintenance of an improved body weight become difficult to achieve. Encouragement for children with simple obesity is best provided by frequent visits (e.g. three‐monthly) by the patient to a clinic or support service with motivated and interested staff. Multidisciplinary services involving community paediatricians, general practitioners, dieticians, psychologists, or psychiatrists may help to improve the outcome. However, there is no point in continuing to encourage attendance in obese patients who do not adhere to suggested interventions and who do not achieve significant weight loss, although intermittent clinical follow‐up to monitor for early signs of the complications of obesity may still be necessary. Although medical therapy, including inhibitors of nutrient absorption from the gut (e.g. acarbose, guar gum, and pancreatic lipase inhibitors), appetite suppressants (e.g. amphetamine derivatives and serotoninergic agents) and agents which increase energy expenditure (e.g. T4 and adrenergic agonists), have been used in the treatment of obesity in adults, there is little experience of their use and efficacy in childhood. Side effects may be problematic and their use is not currently recommended in children. There has also been recent interest in the potential benefits of metformin though results of randomized trials have proved disappointing and treatment of obesity in the absence of type 2 diabetes with metformin is not routinely advised. There is increasing interest in the value of bariatric surgical interventions, such as laparoscopic gastric banding or the Roux‐en‐Y gastric bypass procedure in extremely obese children. When undertaken in children, dramatic amounts of weight loss can be achieved although such children will require careful monitoring for side effects in specialist clinical services. Where excessive weight is the consequence of an underlying endocrinopathy, treatment of hormone deficiency with the relevant hormone replacement (e.g. GH or T4) should result in a decrease in body fat. There is evidence that Prader–Willi syndrome is associated with hypothalamic dysfunction, including impaired GH secretion. GH therapy (0.25 mg/kg per week to a maximum of 2.7 mg daily) is now widely used for the improvement of growth and body composition in children with Prader–Willi syndrome, providing there is no evidence of upper airway obstruction or severe obesity (weight exceeding 200% of ideal for height) and pre‐treatment sleep studies do not demonstrate any evidence of sleep apnoea. Specific medical or surgical interventions for hyperinsulinism or Cushing’s syndrome will also lead to significant weight loss. Obese teenage patients who have significant comorbidities, such as type 2 diabetes or other metabolic abnormalities or those experiencing significant adverse effects on cardio‐respiratory function, will require referral to adult services in their mid‐to‐late teenage years. Seeing these patients in a joint clinic staffed by both adult and paediatric specialists may be especially helpful to the paediatrician as it is likely that his/her adult colleagues will have much greater experience of a range of therapeutic interventions relevant to the management of the obese individual, some of which may be of value in the teenage age group.
Obesity
Physiology
Definition
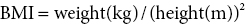
Aetiology
Risk factor
Examples
Genetic
Obesity in either or both parents
Early adiposity rebound
Environmental
Socio‐economic deprivation
Single child
Single parent
Diet‐related
Bottle‐fed in infancy
High fat diet
Disorganized eating patterns
Activity‐related
Physical inactivity
Increased television watching
Short sleep duration
Cause
Examples
Syndromes
Laurence–Moon–Biedl syndrome
Down’s syndrome
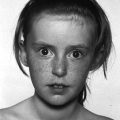
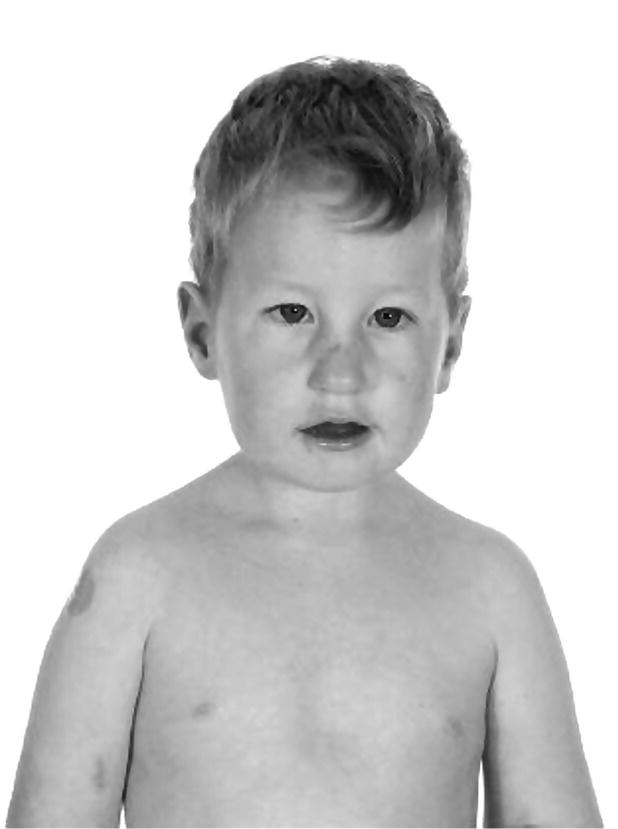
Prader–Willi syndrome (Figure 11.2)
Single gene mutations
Melanocortin‐4 receptor
Proopiomelanocortin
Leptin
Leptin receptor
GNAS (pseudohypoparathyroidism)
Hypothalamic damage
Trauma
Tumours, e.g. craniopharyngioma
Post encephalitis
Endocrine abnormalities
Growth hormone deficiency
Hypothyroidism
Cushing’s syndrome
Hyperinsulinism
Immobility
Spina bifida
Cerebral palsy
Impaired skeletal growth
Achondroplasia
Drugs
Insulin
Steroids
Antithyroid drugs
Sodium valproate
Antipsychotics e.g. risperidone
Regulation of body fat
Factors which increase food intake
Factors which decrease food intake
Noradrenaline
Serotonin
Opioids (e.g. dynorphin and β‐endorphin)
Dopamine
Ghrelin
Cholecystokinin
Galanin
Corticotrophin‐releasing factor
Neuropeptide Y
Neurotensin
Melanin‐concentrating hormone
Bombesin
Agouti‐related peptide
Calcitonin gene‐related peptide
Orexins A and B (hypocretins 1 and 2)
Thyrotropin‐releasing hormone
Growth hormone‐releasing hormone
Amylin
Adrenomedullin
Peptide YY3−36
Leptin
Glucagon
Glucagon‐like peptide 1
α‐Melanocyte stimulating hormone
Cocaine‐ and amphetamine‐related peptide
Somatostatin
Preliminary examination and investigation
History
Examination
Clinical sign
Possible diagnosis
Acanthosis nigricans
Type 2 diabetes (insulin resistance)
Hypertension
Syndrome X (metabolic syndrome)
Dysmorphic features (see Table 11.5)
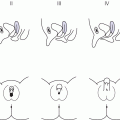
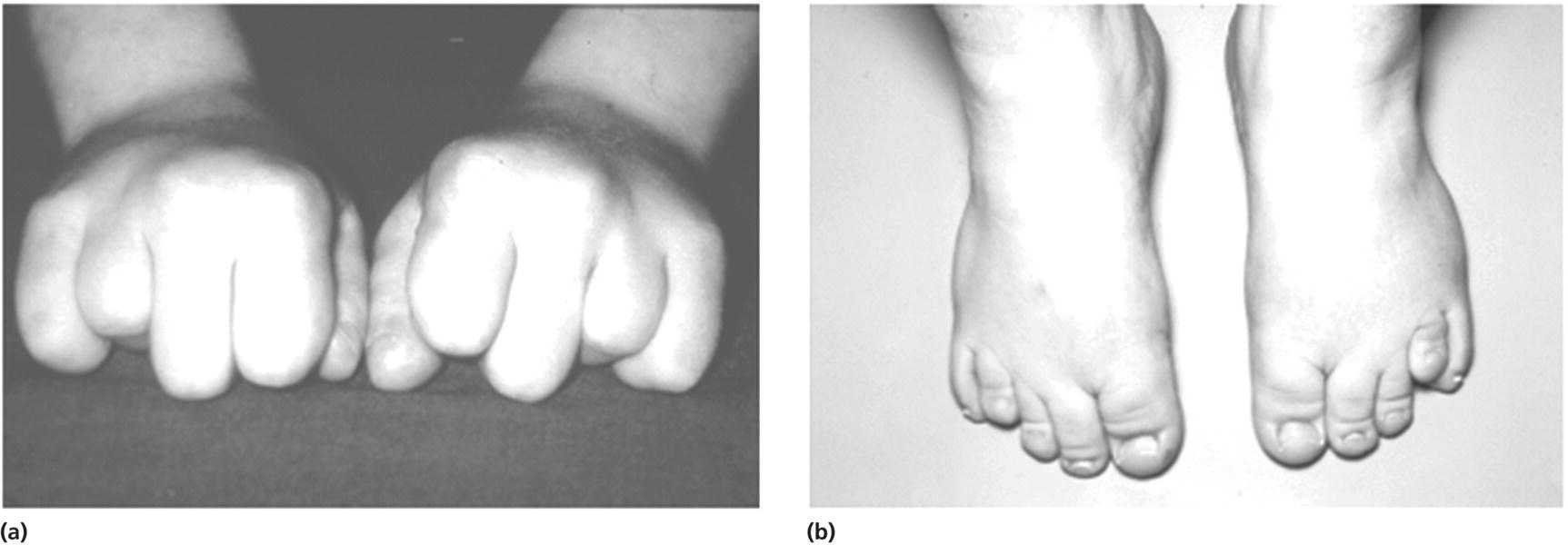
Laurence–Moon–Biedl syndrome (Figure 11.3)
Prader–Willi syndrome (Figure 11.2)
Pseudohypoparathyroidism (Figure 11.4)
Beckwith–Wiedemann syndrome
Impaired visual fields
Intracranial tumour, e.g. craniopharyngioma
Papilloedema or optic atrophy
Tall stature or increased height velocity
Simple obesity, hyperinsulinism
Short stature or decreased height velocity
Growth hormone deficiency, hypothyroidism, Laurence–Moon–Biedl syndrome
Prader–Willi syndrome
Pseudohypoparathyroidism
Cranial midline defects (e.g. cleft lip and palate)
Growth hormone deficiency
Truncal obesity
Goitre
Hypothyroidism
Prolonged ankle tendon reflexes
Proximal myopathy
Cushing’s syndrome
Hypertension
Truncal obesity
Abnormal gait
Spina bifida
Cerebral palsy 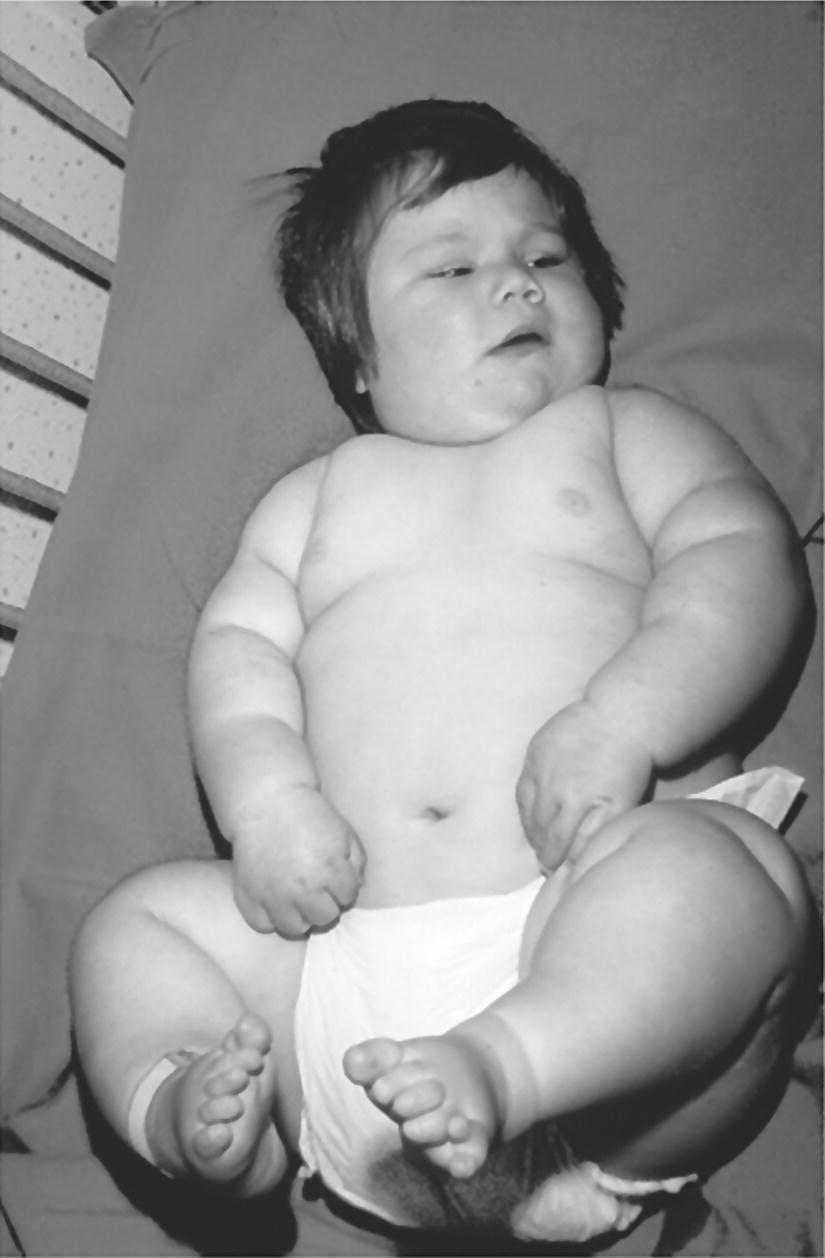
System
Prader–Willi syndrome
Laurence–Moon–Biedl syndrome
Pseudohypo‐parathyroidism
Facial
Narrow forehead
Squint
Round facies
Olive‐shaped eyes
Short neck
Antimongoloid slant
Epicanthic folds
Squint
Carp mouth
Micrognathia
Abnormal ear lobes
Skeletal
Short stature
Short stature
Short stature
Small hands and feet
Polydactyly
Shortened fourth metacarpals and metatarsals
Clinodactyly
Clinodactyly
Syndactyly
Scoliosis
Dislocated hips
Neurosensory development and behaviour
↓ IQ
↓ IQ
↓ IQ
Hypotonia and feeding problems in infancy
Retinitis pigmentosa
Insatiable appetite
Uncontrollable rage
Endocrine
Hypogonadotrophic hypogonadism
Hypogonadotrophic hypogonadism
PTH‐resistant hypocalcaemia
Type 2 diabetes
Diabetes insipidus
Other
Renal anomalies
Subcutaneous calcifications
Complications
System
Adverse effect
Metabolic and endocrine
Hyperinsulinaemia
Impaired glucose tolerance
Type 2 diabetes
Hyperlipidaemia
Metabolic syndrome
Advanced pubertal development
Polycystic ovarian disease
Non‐alcoholic steatohepatitis (NASH)
Cholelithiasis
Cardiovascular
Hypertension
Respiratory
Breathless on exertion
Obstructive sleep apnoea
Pickwickian syndrome
Skeletal
Knock knee or bow legs
Slipped capital femoral epiphysis
Skin
Furunculosis
Intertrigo
Neurological
Idiopathic intracranial hypertension
Psychological
Poor self‐image
Bullying
Behavioural problems
Anxiety
Depression
Investigations
Treatment
When to involve a specialist centre
Future developments
Transition
Controversial points
Potential pitfalls
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree