Chapter 5
Nutrition in Critically Ill Children
Rosan Meyer and Luise Marino
Introduction
Data from the Paediatric Intensive Care Audit Network in the UK indicate that only 1.1%–7.7% of children are admitted to a paediatric intensive care unit (PICU), with the majority of those children (47%) being below 1 year of age [1]. Nutritional management of critically ill children (CIC) is therefore a very specialist and challenging area in paediatric practice. In addition to minimising the effects of starvation associated with suboptimal nutrition and preventing nutritional deficiencies and excesses, its goal is to sustain organ function and prevent dysfunction of the cardiovascular, respiratory and immune systems until the acute-phase inflammatory response resolves (Table 5.1) [2]. Both undernutrition and overnutrition have the potential to compromise this goal and significantly complicate and increase the length of hospital stay [3–5]. Overfeeding can lead to increased carbon dioxide production, resulting in difficulties with weaning from mechanical ventilator support, fatty liver, as well as diarrhoea associated with electrolyte imbalances and other well documented metabolic and physiological complications [6]. Underfeeding, however, is a more common occurrence in PICUs that has not improved over the last 30 years, in spite of great medical advances. Pollack et al. [3] found in 1981 that 16%–20% of critically ill children develop significant, acute protein energy malnutrition (PEM) within 48 hours of admission to a PICU; in 2006, Hulst et al. found that 24% of children have PEM [7]. An even higher prevalence of moderate severe malnutrition was found by Delgado et al. [8] in 2008, with 53% of the children suffering from PEM. This has a significant effect on muscle strength [9], it reduces wound healing due to altered immunity and increases rates of sepsis [7]. Ensuring optimal nutritional support in critically ill children is therefore crucial.
Table 5.1 The effect of critical illness on the major organs
| Gastrointestinal system | Cardiovascular system | Respiratory system | Renal system |
|
|
|
|
Inflammatory and metabolic responses that impact on nutritional requirements
Endocrine and inflammatory response
A knowledge of metabolic changes and fuel utilisation during physiological stress can assist dietitians in commencing nutritional support at the appropriate time and suggesting a suitable feeding route and feed composition.
Critical illness is characterised by a cascade of endocrine and metabolic reactions, affecting all major organs (Fig. 5.1). The reaction of the body to physiological stress changes over time. The acute phase response can be divided into the ebb phase and flow phase. The ebb phase is characterised by the body’s attempt to maintain normal perfusion and mobilisation of stress hormones; the flow phase is the dynamic state of acute injury and affects substrate (protein, carbohydrate and fat) metabolism. The final phase, the anabolic phase, is characterised by the slow re-accumulation of protein and body fat after the metabolic response to injury subsides [10–12].
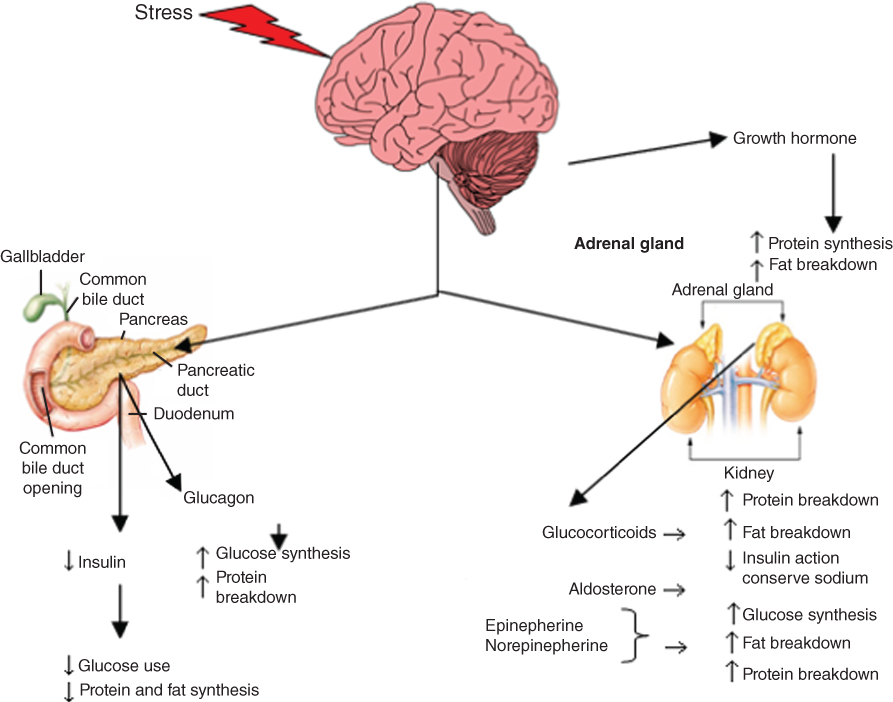
Figure 5.1 Endocrine and metabolic cascade in critically ill children [13, 14, 18, 114]. Source: Adapted from Krause MV, Mahan LK, Arlin M Physiological stress: trauma, sepsis, burns and surgery. In: Krause’s Food, Nutrition and Diet Therapy, 10th edn. Philadelphia: WB Saunders Company, 2002, p. 492. Reprinted with permission of Elsevier.
During critical illness immune cells, e.g. macrophages, lymphocytes and neutrophils, regulate the inflammatory response through the release of cytokines [in particular interleukins (IL) such as IL-1, IL-6, IL-8, IL-10 and tumour necrosis factor α (TNFα)] and chemokines such as heat shock protein 70. All are important mediators of the stress response (Table 5.2). Cytokines signal through receptors on the surface of target cells and organs mediating the following responses:
- upregulation or downregulation of gene expression influencing wound healing and immunocompetence
- release of counter-regulatory hormones
- cell to cell signalling orchestrating the inflammatory response which affects substrate metabolism [13–15].
Table 5.2 Cytokines involved in the acute phase of injury and possible effects on substrate metabolism and nutrition
| Cytokines | Relevant function | Interaction between metabolism, nutrition and cytokines |
| Pro-inflammatory cytokines | ||
| TNFα [13, 15, 104] | Pro-inflammatory; release of leucocytes by bone marrow; activation of leucocytes and endothelial cells | Increases glucose transport and impacts on muscle lipolysis |
| IL-1 [13, 17] | Involved in pyrexia; T-cell and macrophage activation | Impact on hepatic and muscle lipogenesis |
| IL-6 [13, 17] | Growth and differentiation of lymphocytes; activation of the acute-phase protein response | Impact on hepatic lipogenesis Increases during multi-organ dysfunction and associated with poor nutritional status |
| IL-8 [13, 15] | Chemotactic for neutrophils and T-cells | Concentrations during the first 24 hours are predictive of worsening organ dysfunction |
| Anti-inflammatory cytokine | ||
| IL-10 [15, 17] | Inhibits immune function | Increases during multi-organ dysfunction and associated with poor nutritional status Both excessive and under expression of IL-10 is negatively associated with outcome Negatively correlated with resting energy expenditure: increase in IL-10 associated with lower energy expenditure Inhibits pro-inflammatory cytokine production by macrophages, monocytes, neutrophils and natural killer cells Attenuates the synthesis of TNF cell surface receptions Controlling cytokine regulating the inflammatory response |
IL, interleukin; TNF, tumour necrosis factor.
Energy metabolism
There has been significant debate over the last 10 years about the impact of acute illness on energy metabolism in children, which differs from that seen in adults. Some older studies have suggested a hypermetabolic state during the acute phase of illness [16]; however, new evidence points towards more children being hypometabolic when it comes to energy expenditure. A recent study by Briassoulis et al. [17] found that on the first day of admission 48.6% of children were hypometabolic (<90% of predicted basal metabolic rate, BMR), 40.5% were normometabolic (90%–110% of predicted BMR) and 10.8% were hypermetabolic (>110% of predicted BMR). A hypermetabolic pattern only emerged after 2 weeks of admission in 60% of children. This unique pattern of metabolism may be explained by a multitude of factors, including the physiological response to stress, improved medical treatment as well as the unique nature of fuel utilisation in critically ill children (Tables 5.2 and 5.3) [11, 12, 18, 19].
Table 5.3 Factors that influence energy expenditure
| Factor | Influence on energy expenditure |
| Sedation [105] | ↓ Energy expenditure (reduced brain activity) |
| Muscle relaxants [20, 105] | ↓ Energy expenditure but not of clinical significance |
| Ventilation (with humidified air) [20] | ↓ Energy expenditure, reduces the workload of breathing as well as heat loss in fully ventilated children, but where ventilatory support is minimal may not have impact |
| Thermoneutral environment [19] | ↓ Insensible losses, therefore lowers energy expenditure |
| Pyrexia [106] | ↑ Energy expenditure (8%–12% increase in energy expenditure per 1°C above normal) |
| Paracetamol/NSAIS [106] | ↓ Energy expenditure as blunts response to fever; clinical relevance questionable |
| Severity of disease [20] | Inconclusive evidence |
| Diagnosis [19, 20] | No difference in energy expenditure in different diagnoses |
| Severity of disease [20] | No significant difference in energy expenditure |
| Movement/activity [42] | ↑ Energy expenditure |
NSAIS, non-steroidal anti-inflammatories.
In addition to the factors listed in Table 5.3 authors have speculated that during the acute phase growth does not take place and this energy is diverted into the recovery processes [19, 20]. This hypothesis is supported by the data from Hulst et al. [21] who found that insulin-like growth factor 1 (an anabolic hormone) remained low until day 4 of PICU admission and T3 levels remained below normal at days 4 and 6 in 88% and 89% of the older children, respectively. Although more research needs to take place before specific guidelines on energy expenditure can be published, it is clear that substrate metabolism varies greatly during admission and that during the early phase of illness hypometabolism is usual, whereas in the later phase of illness hypermetabolism predominates. This needs to be taken into account when calculating energy requirements and choosing feeds for these patients, which may require changes being made to prescribed energy intake during the course of a PICU stay.
Substrate utilisation
Several studies have focused on the relation between CIC’s metabolic state, their nutritional intake, substrate utilisation and nitrogen balance. In a similar way to critically ill adults the stress response and the severity of disease are characterised by protein catabolism. In contrast to adults, fat is the preferred fuel in children and is readily oxidised. Conversely carbohydrates are poorly utilised during critical illness. Gluconeogenesis is also a characteristic of paediatric critical illness and muscle mass is rapidly depleted of amino acids, such as glutamine, which are utilised in de novo glucose production [22, 23]. Maximal glucose oxidation occurs at a glucose intake of 5 mg/kg/min. If intake exceeds 8 mg/kg/min lipogenesis takes place, leading to an increase in triglyceride levels, fat deposition in the liver and an increase in fat instead of lean body mass [18]. It is therefore important to ensure that carbohydrate intake in CIC is monitored, especially if parenteral nutrition (PN) is used.
Mechanism for muscle wasting
Sepsis associated muscle wasting is well described in the adult intensive care unit (ICU), although the prevalence is not known in children. Causative factors include sepsis, muscle disuse, fasting, cancer, cardiac failure and renal dysfunction. In ICU there is often prolonged bed rest or immobilisation, use of sedatives and neuromuscular blockades, acute or chronic organ dysfunction, medication using steroids [24] and increased levels of cytokines, e.g. IL-6, IL-10, TNFα (which are associated with muscle degradation), amongst other factors [15, 24] (Fig. 5.2).
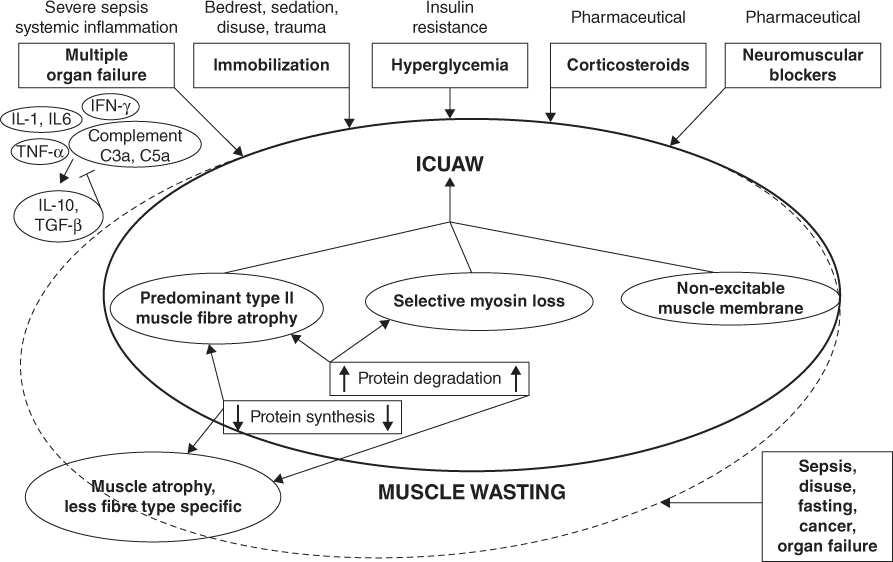
Figure 5.2 Risk factors for muscle wasting and ICU associated wasting from Schefold et al. with permission [24]. ICUAW, intensive care unit acquired weakness. Reprinted with permission of Springer.
There is often an imbalance between anabolism and catabolism with protein turnover outstripping protein synthesis leading to a net loss of muscle mass which occurs as a result of de novo gluconeogenesis [22, 24]. Muscle wasting and ICU induced myopathy has not been described in children and it may be that the pathophysiology of paediatric critical illness does not result in this phenomenon. However, as a significant number of children admitted to PICU develop malnutrition it is likely that muscle wasting also occurs in children.
Nutritional assessment
Growth failure is common amongst children admitted to PICU. It is therefore imperative to classify the type of growth impairment (i.e. wasting, stunting or faltering growth) to enable a targeted nutritional care plan. Malnutrition in this population is multifactorial and may be illness and non-illness related and it is important to identify the contributors (Fig. 5.3). The multifactorial nature of malnutrition in this population makes nutritional assessment particularly challenging [25].
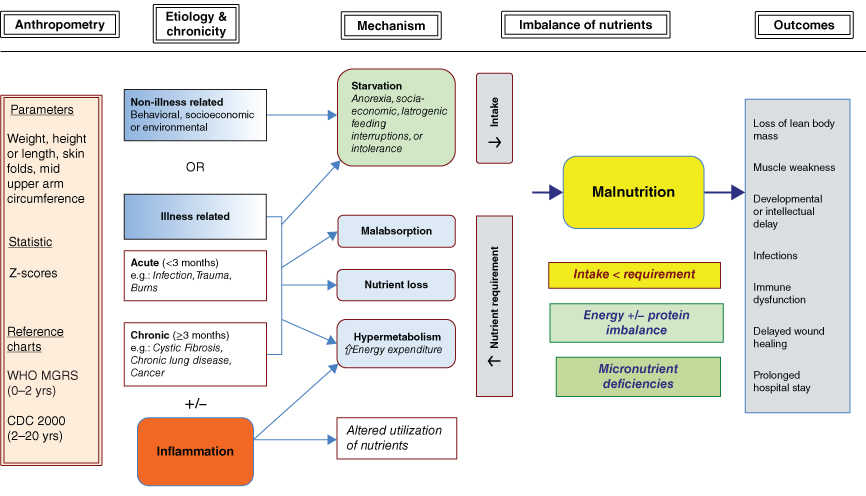
Figure 5.3 Aetiology of illness and non-illness related malnutrition in CIC [25] (Reprinted with permission of Sage).
Anthropometry, biochemical markers, clinical and dietary review form part of the nutritional assessment in CIC [26]. This process, however, is notoriously difficult due to a multitude of factors including oedema, ascites and severity of disease, which often makes it challenging to obtain an accurate body weight. In addition, the emotional impact of having a child in the PICU frequently makes the diet history from the carers unreliable.
A recent UK study found that only 20.5% of CIC had an accurate admission weight documented [27] so it is quite common practice to estimate their weight. In the past the Advanced Paediatric Life Support formula [(age + 4) × 2] was used [28]; however, Luscombe et al. [27, 29] found that this formula underestimates the weight significantly and that a new formula [weight = 3(age) + 7] allows for a more safe and accurate estimation. A formula, however, can never replace an accurate weight measurement, as it is used not only in the assessment of nutritional status and for calculating nutritional requirements but also for estimating fluid requirements and medication doses. Transfer and hospital notes as well as the child’s personal health record (red/blue book) may have a recent accurate weight (often height and head circumference as well), which may be useful. It is important that available accurate weight, height and head circumference is plotted on an appropriate growth chart [26, 30].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree