Nutrition and Aging: Introduction
Throughout life, nutrition is an important determinant of health, physical and cognitive function, vitality, overall quality of life, and longevity. The quantity and variety of available foods, as well as the meaningfulness of the social interactions provided by meals, are important to psychological well-being. The composition of the diet and the amount that is consumed are strongly linked to physiological function. When a well-balanced diet is not maintained, malnutrition may develop with consequent detrimental effects on health and well-being.
Malnutrition can have many manifestations. As outlined in Chapter 40, a diet that is deficient in one or more required nutrients (e.g., calories, protein, minerals, fiber, or vitamins) can lead to a state of nutritional deficiency. The greater the magnitude and duration of the nutritional deprivation and the more fragile the individual, the more likely nutritional deficits will produce noticeable body compositional changes, functional impairments, or overt disease. Even borderline dietary deficiencies can have important health consequences such as producing subtle organ system impairments, diminished vitality, or increasing the individual’s susceptibility to disease. Protein and protein-energy undernutrition are two of the most common, frequently unrecognized, and potentially serious forms of nutritional deficiency. The prevalence of these conditions is particularly high among chronically ill older individuals and those in hospitals, nursing homes, and other institutional settings. Although there is a complex interrelationship between nutrition, disease, and clinical outcomes, protein and protein-energy undernutrition appear to be significant contributors to disease-related morbidity and mortality in these populations. At the other end of the spectrum, the persistent consumption of excess quantities of one or more nutrients can have similar untoward consequences. Forms of malnutrition that result from excess consumption include hypercholesterolemia, hypervitaminosis, and obesity. Studies indicate that obesity is the most common nutritional disorder of advanced age in western societies with a high prevalence among the noninstitutionalized free-living elderly. Many obese older individuals have other nutritional disorders. Among chronically ill or functionally debilitated obese older individuals, protein undernutrition is a common, serious, and frequently unrecognized problem that can develop for many reasons including an imbalanced diet, disease, and inactivity.
Recognizing and maintaining an optimally balanced diet is an important challenge, particularly as individuals age. The challenge is particularly great for older people who already are malnourished, especially if they have nutritional disorders that developed earlier in life, such as obesity, osteoporosis, or protein undernutrition. Even healthy individuals often fail to maintain an optimal diet owing to lack of knowledge, resources, or willpower. The process of aging can introduce other factors including acute and chronic disease, physical disabilities, social isolation, use of multiple medications, depression, impaired cognitive ability, and disregulation of appetite control that may contribute further to poor eating habits and the development or exacerbation of nutritional disorders. In turn, inappropriate dietary intake and poor nutritional status can impact the progression of many acute and chronic diseases such as coronary heart disease, cancer, stroke, diabetes, and osteoporosis, which are among the 10 leading causes of death in the United States. The 1988 Surgeon General’s Report on Nutrition and Health noted that two-thirds of all deaths within the United States are because of diseases associated with poor diets and dietary habits.
Assessing the quality of the diet of elderly persons is critical to addressing issues relevant to their health and nutritional status. Such an assessment must be based on knowledge of what constitutes a balanced diet for a given individual. The goal of this chapter is to identify an approach to nutrition evaluation and management that takes into account the unique needs, limitations, and desires of each elderly individual. The chapter starts out by examining the interrelationship among nutrition, activity, disease burden, and health outcomes and then focuses on age-related changes in body composition, lifestyle, and appetite regulation that affect nutritional status and nutrient requirements. Also included is a discussion of specific dietary considerations related to optimal health requirements.
The Interrelationship between Nutrition, Activity, and Disease
Although nutrition is a vital component of good health, it cannot be evaluated in isolation. The relationship between nutrient intake and health is influenced by other factors, most notably activity level, disease burden, and advancing age. A basic understanding of these interrelationships is essential in order to assess the potential benefits and limitations of nutritional interventions.
Nutrition and physical activity are closely linked, each having vitally important and interacting effects on body composition, functional ability, and well-being. The balance between nutrient intake and physical activity is particularly important in determining muscle mass and strength, body fat content and distribution, and bone density and resilience. In recognition of the importance of the interrelationship between nutrition and activity on health, the U.S. Department of Agriculture (USDA) has released the MyPyramid food and activity guidance system. The MyPyramid symbol (Figure 38-1), which replaces the prior food pyramid, is designed to emphasize this interrelationship. The symbol is also designed to convey the message that variety, proportionality, moderation, gradual improvement, and personalization are all important in both diet and exercise prescriptions. Consumers can go to the MyPyramid Web site (www.mypyramid.gov) to learn more about this system and to obtain a detailed assessment and analysis of their current eating and physical activity habits.
To preserve existing muscle mass and strength, it is necessary to maintain both an adequate level of physical activity and a balanced diet that includes sufficient protein, energy, vitamins, and minerals to meet metabolic demands and prevent negative nitrogen balance (as discussed in detail below). It is not known precisely what level of physical activity is needed to prevent loss of existing muscle mass and strength in older adults. However, studies indicate that even a week or two of bed rest or similar degrees of activity restriction can result in noticeable loss of muscle mass, strength, and function even when the diet is adequate and the individual is otherwise healthy. In one study of 12 healthy, moderately active older adults, 10 days of voluntary total bed rest resulted in a 16% loss of strength and a 6% loss of skeletal muscle mass from the lower extremities. Muscle biopsies indicated that muscle protein synthesis declined by 30%. Despite the provision of a diet containing the recommended dietary allowance for protein, the participants remained in negative nitrogen balance throughout the study. In contrast, fat mass did not change. The combination of inadequate diet and inactivity can result in an even more rapid loss of muscle. In contrast, overfeeding does not prevent muscle atrophy associated with inactivity and may exacerbate the functional consequences since the excess nutrients are converted to fat.
To increase muscle strength, size, or endurance, the average daily level of exertion has to increase significantly. Aerobic exercises are most effective in improving the oxidative capacity of muscles and are the mainstay of endurance training. High-intensity, progressive resistance training, such as weight lifting, is needed to build strength and mass. Nutrition alone has never been demonstrated to be an effective method of repleting muscle mass, improving strength, or increasing endurance in frail older individuals who have experienced a recent loss of weight. Efforts at repletion should focus on both increasing nutrient intake and exercise. Based on studies of healthy elderly men, the combination of progressive resistance muscle strength training and a high protein diet (containing up to 1.6 g of protein/kg body weight/d) may be the most effective method of improving muscle mass.
Exercise can have an important effect on body fat content and distribution. In obese older individuals, exercise can play a synergistic role with caloric restriction in promoting weight loss and preventing further weight gain. With weight loss, visceral fat is mobilized at a rate two to five times that of other fat stores. Even when total body weight does not change, exercise can induce a significant decrease in intra-abdominal fat in both obese and nonobese individuals. The preferential mobilization of fat stores has important metabolic implications for the prevention or treatment of the insulin resistance syndrome, since it is predominantly excess visceral fat that is associated with the derangements of dyslipidemia, elevated fibrinogen, hyperinsulinemia, and hypertension.
Exercise and nutrition also play a critical role in maintenance of optimal bone density and strength. As discussed in Chapter 117, the nutrient needs of bone include the correct balance of protein and energy and adequate intake of vitamins and minerals, especially vitamin D and calcium. The amount of exercise that is needed for optimal bone health is not defined. However, it is known that people who exercise regularly have higher bone density than more sedentary age-, race-, and gender-matched controls. Randomized controlled trials involving postmenopausal women demonstrate that those assigned to progressive resistance muscle strength training or weight-loading aerobic exercise programs attain a 1% to 1.6% greater bone mineral density in both the lumbar spine and femoral neck region per year compared to the controls. It is also known that bed rest and weightlessness are associated with a rapid decline in bone mineral density. Consequently, osteopenia can develop despite an optimal diet if exercise or other weight bearing activities are not adequate. Because of its apparent beneficial effects on bone mineral density, exercise should be combined with an appropriate diet for both prevention and treatment of osteoporosis and fracture-related disability.
Having both direct and indirect effects on numerous metabolic processes within muscle, bone, and adipose tissues, exercise has a major impact on how nutrients are utilized by the body during health and illness. By inducing an increase in the mass and metabolic capacity of muscle, exercise affects energy expenditure, glucose metabolism, and size of protein reserves in a manner that counteracts some of the effects of aging and thus has important nutritional implications for individuals as they grow older. Total energy expenditure (TEE) represents the sum of basal energy expenditure, postprandial thermogenesis, and the energy expenditure of activity. Muscle represents not only the primary source of energy expenditure during physical activity, it is also the primary contributor to basal energy expenditure, which may represent 50% to 80% of TEE. With advancing age, there is a parallel decline in muscle mass and both basal and total daily energy expenditure that may be partially or fully accounted for by the fact that people tend to become more sedentary as they grow older. A study of master athletes demonstrates that men who maintain a vigorous weekly routine of weight training throughout their lives have the muscle mass and energy capacity comparable to that of healthy 30-year-old males. Nonexercising older men have significantly less muscle mass than their younger counterparts.
Exercise-induced increases in muscle size or protein content result in greater body protein reserves, which can be critical to survival during episodes of nutritional deprivation that usually accompany profound physiologic stress such as that caused by trauma, sepsis, or other acute disease. Such acute physiologic insults trigger an acute inflammatory response that causes ketogenesis to be suppressed, leaving glucose as the primary energy source available to the body. The problem is invariably compounded by reduced nutrient intake that results as a consequence of the anorexia and gastrointestinal tract dysfunction induced by the inflammatory response. With nutrient intake suppressed, gluconeogenesis becomes the predominant source of glucose. Since the substrate for gluconeogenesis is provided by catabolism of skeletal muscle, lean body mass becomes an important determinant of survival. Once lean body mass falls below a critical level, the chance of surviving a serious acute illness diminishes dramatically. Studies conducted within the Warsaw Ghetto, hospital intensive care units, and other settings suggest that a loss of >40% of baseline lean mass is incompatible with life. Other studies indicate that very few healthy people have a lean body mass that is <70% of the mean for that of adults aged 20 to 30 years.
In addition to inducing muscle hypertrophy, exercise also affects insulin sensitivity, glucose disposal, and HDL levels directly and plays a synergistic role with diet in maintaining a healthy weight and a sense of well-being. These effects of exercise can be important adjuncts to good nutrition in the prevention and treatment of hypertension, diabetes, dyslipidemia, and osteoporosis.
There is a complex interrelationship between nutrition, health status, and clinical outcomes. Although a full discussion of this topic is beyond the scope of this chapter, it is important to emphasize several key points. First, nutrient requirements and the ability to metabolize select nutrients are influenced by many disease states. In addition, many diseases compromise the older individual’s ability to consume adequate amounts of all nutrients. This can occur through a number of mechanisms including disease-induced suppression of appetite, alteration of the normal swallowing mechanism, maldigestion or malabsorption, and a loss of self-feeding ability.
The detrimental effects of disease on nutrient metabolism often become more pronounced with advancing age. This is particularly true of the many acute and chronic diseases that induce an inflammatory response, including acute and chronic infections, congestive heart failure, chronic pulmonary disease, cancer, end-stage renal disease, and rheumatoid arthritis. With advancing age, the inflammatory response often becomes dysregulated as indicated by persistently elevated serum concentrations of proinflammatory cytokines and other inflammatory mediators (Chapter 4). The proinflammatory cytokines include interleukin(IL)-6, IL-1(beta), tumor necrosis factor(TNF)-alpha, and possibly IL-8 and others. These cytokines function both as intermediaries and directly to induce many of the signs and symptoms associated with inflammation including weight loss. The proinflammatory cytokines have been implicated in the pathogenesis of many of the detrimental consequences of chronic inflammation including anemia, hypoalbuminemia, and cachexia. IL-1, IL-6, and TNF-alpha all contribute to the loss of skeletal muscle, fat tissue, and bone mass that characterizes inflammation-associated cachexia. Although anorexia is almost always a contributing factor, the inflammation-induced loss of fat and lean mass is often refractory to nutrition support. Proinflammatory cytokines create a state of muscle catabolism by suppressing muscle protein synthesis and/or accelerating muscle protein breakdown independent of dietary factors. Additionally, the proinflammatory cytokines induce lipolysis while suppressing fat and liver lipoprotein lipase activity resulting in hypertriglyceridemia and a decrease in the availability of fat to be used as an energy source. As energy production from fat diminishes, the importance of glucose as an energy source increases. The amino acids derived from the muscle proteolysis are either converted to glucose or are consumed in hepatic synthesis of acute phase proteins. Additionally, cytokines stimulate the release of cortisol resulting in a further acceleration of the muscle catabolism. Since these potentially deleterious effects of disease can be difficult to predict, older individuals with one or more acute or chronic health problems should have frequent reassessments of their nutritional status and their nutritional care plan revised as necessary. Although nutrient intake may not be adequate to completely reverse inflammation-induced catabolism, a low nutrient intake will accelerate the development of cachexia. Optimally, good nutritional care should be part of the overall plan of medical intervention aimed at treating the underlying pathology as well as addressing protein and energy deficits. Although a number of specific nutrients are being studied to determine their value in counteracting inflammation-induced loss of lean body mass, there is not yet adequate evidence that any given dietary supplement is more effective than current standard dietary or nutrition support practices.
Age–Related Changes that Affect Nutrition
With advancing age, there are significant changes in body composition that affect the nutritional needs of an individual. Based primarily on cross-sectional studies, weight increases steadily in most people from age 30 to 60 years. An increase in total body fat accounts for a majority of this weight gain. After age 60, weight usually stabilizes, and then begins to decline. Improved survival of nonobese individuals during middle age and cohort effects may account for some of the decline in weight with age that is reported in the cross-sectional studies. However, weight maintenance becomes increasingly difficult in the advanced years of life. The incidence as well as the potential causes of weight loss increases with age, particularly beyond age 75.
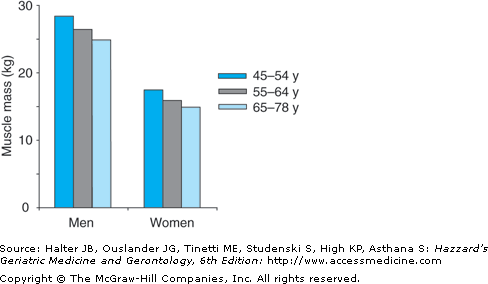
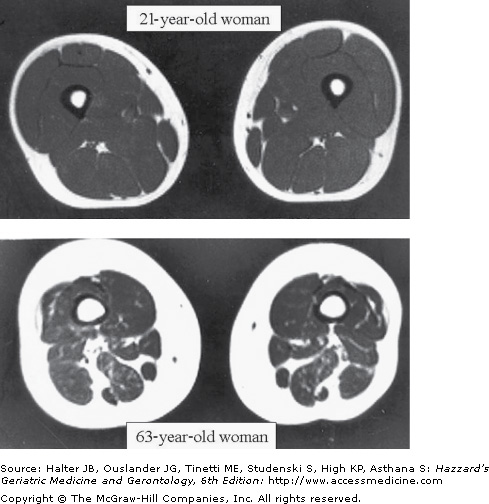
Regardless of whether or not weight changes, advancing age is characterized by a progressive loss in lean body mass, a relative increase in fat mass, and a redistribution of fat from peripheral to central locations within the body. Based primarily on cross-sectional studies, it appears that all of these changes begin in the third decade and increase at an accelerated rate after age 65. This late accelerated phase may be a threshold effect brought about by the loss of lean body mass and the increased prevalence of chronic disease in old age. The loss of lean body mass consists predominantly of skeletal muscle, particularly type II or fast twitch fibers. Central lean body mass, such as the liver and other splanchnic organs, is relatively preserved. Some studies indicate that muscle mass may decline by up to 45% between the third and eighth decade of life (Figures 38-2 and 38-3). The few longitudinal studies that have been reported indicate that the loss of muscle mass with age may be greater in men than women. However, this remains controversial.
The loss of muscle mass with age appears to be the result of multiple interrelated factors including age-related changes in metabolism, function, or structure of organ tissues, disease, medical therapeutics, heritability, and behavior and lifestyle choices of the individual. Notable age-related changes that appear to contribute to loss of muscle include a progressive loss of alpha motor units in the spinal column, a diminution in the intrinsic muscle protein synthesis capacity, and a decline in the production of multiple hormones including testosterone, estrogen, and the insulin-like growth factors. The decline in the intrinsic muscle protein synthesis capacity parallels and may be causally related to a loss of mitochondrial ATP production with advancing age. Other age-related changes detrimental to muscle include the increased production and delayed inactivation of catabolic mediators and a change in liver protein metabolism that may decrease the availability of amino acids to axial muscles. The decline in nutrient consumption and activity level that often accompanies advanced age are possibly modifiable contributors to the loss of muscle mass. Many diseases accelerate the age-related decline, particularly degenerative diseases of the central nervous system and those affecting any part of the motor pathway. An accelerated loss of muscle mass can occur when a serious illness requires treatment with steroids or other antianabolic drugs or is accompanied by low nutrient intake and the need for prolonged bed rest. In all individuals, the loss of muscle mass with age is closely linked with a reduction in muscle strength and exercise capacity, a decline in one causing further loss of the others. When exercise capacity falls below that required to comfortably perform basic activities of daily living, physical activity often becomes very limited, which causes a rapid acceleration of the downward spiral. The loss of muscle mass and exercise capacity is also linked to the development of coronary artery disease, diabetes mellitus, and other diseases that contribute further to the decline.
In parallel with the loss of lean body mass, there is an increase in the relative amount and the distribution of body fat with advancing age. Between the second and ninth decades of life, the percentage of body weight that is fat increases by 35% to 50% in females and to an even greater extent in males. In one cross-sectional study of 500 healthy individuals between the ages of 18 and 85 years, fat mass as a percentage of body weight increased across the age range of the sample from 33% to 44% in females and 18% to 36% in males. Whether or not total body weight changes, intra-abdominal (visceral) fat increases quantitatively and proportionally more than peripheral fat mass. In females, the accumulation of intra-abdominal fat accelerates at menopause and represents primarily a shift from peripheral sites. In males, the increase in intra-abdominal fat with age represents primarily an increase in total body fat mass. For a given waist circumference, older adults have greater visceral fat than young adults and men have greater visceral and less subcutaneous fat than women.
Numerous factors have been sited as potential contributors to the changes in adipose tissues with advancing age. Some studies suggest that much of this change can be accounted for by the reduction in both the quantity and oxidative capacity of skeletal muscle that also occurs during the same time interval. With advancing age, fat oxidation is reduced at rest, following a meal, and during exercise. This reduced rate of fat metabolism with age promotes accumulation of fat at both peripheral and central locations. The decline in total skeletal muscle mass that occurs with age correlates with, and may be responsible for, the decreased rate of metabolism of fat at rest. Likewise, the decline in maximal oxidative capacity of muscle may cause the reduction in fat metabolism with exercise. The decline in physical activity resulting from the loss of muscle with age contributes to the increase in intra-abdominal fat. Other factors that may also contribute to the increased intra-abdominal fat with age include declining testosterone in men, estrogen withdrawal or increased testosterone in females, the decline in growth hormone, increased resistance to leptin, and increased secretion of cortisol.
Weight cycling can also contribute to the relative increase in fat mass in some older adults. When older adults lose weight, particularly when this weight loss occurs in association with an acute illness, they usually experience a greater decline in lean compared to fat mass. Those individuals who later regain some or all of the lost weight usually gain predominantly fat mass. It is not known whether exercise, specialized diets, or anabolic agents would be effective in helping to restore lean body mass in these older adults. Given the high prevalence of illness-induced weight loss among older adults, this is an important area for further research.
Maintenance of a stable weight requires a steady balance between nutrient intake and energy expenditure. With advancing age, the metabolic, neural, and humoral pathways that normally maintain this delicate balance by regulating appetite and hunger begin to lose their compensatory responsiveness to changes in energy demands. Psychological, socioeconomic, and cultural influences and numerous disease processes further contribute to the disregulation. From the third to seventh decade of life, these factors integrate to create an imbalance usually favoring a tendency toward weight gain and increased fat deposition, at least in societies where food is plentiful and the physical demands of life are light. However, after age 70, the risk of losing weight increases steadily with each year of survival. This correlates with the findings from numerous studies that indicate that low dietary energy intake is common among both healthy and frail elderly people.
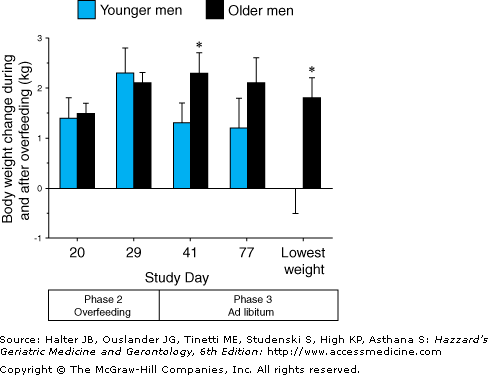
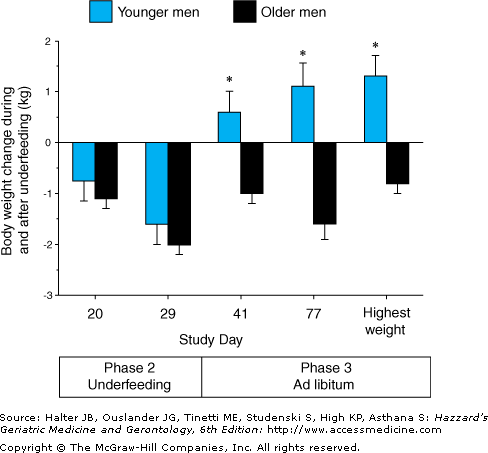
Impaired regulation of food intake has been observed in sedentary elderly men. As shown in Figure 38-4, young and older subjects gained similar amounts of weight during overfeeding. When allowed to resume a normal ad libitum diet, the young subjects decreased their energy intake and lost weight. In contrast, the elderly subjects failed to readjust their nutrient intake and at the end of the study remained above their starting weight. As shown in Figure 38-5, there were similar age group differences in the response to underfeeding. The young and old subjects experienced similar amounts of weight loss during the intervention phase. However, the young men increased their nutrient intake and regained weight after returning to an ad libitum diet, whereas the elderly subjects did not. These findings suggest that a reduced ability to regulate food intake may contribute to the progressive loss of weight that many older individuals experience when their lives are disrupted by illness, psychological stress, or economic hardship.
Figure 38-4.
Body weight change during 21 days of overfeeding by 1000 kcal/d (phase 2) and a subsequent 46-d period of ad libitum diet (phase 3). Values are means (+SEM) for younger (n = 7) and older (n = 9) men. The asterisks indicate significance at P < 0.05 relative to the younger men. (Reproduced with permission from Roberts SB, Fuss P, et al. Control of food intake in older men. JAMA. 1994;272:1601.)
Figure 38-5.
Body weight change during 21 days of underfeeding by 792 kcal/d (phase 2) and a subsequent 46-day period of ad libitum diet. Values are means (+ SEM) for younger and older men. The asterisks indicate significance at P < 0.001 relative to the younger men. (Reproduced with permission from Roberts SB, Fuss P, et al. Control of food intake in older men. JAMA. 1994;272:1601.)
Numerous pathologic and age-related physiologic changes contribute to the difficulty older people have maintaining a balance between metabolic needs and nutrient intake. The look, smell, taste, and texture of food all contribute to the desirability of a meal and can serve to stimulate or inhibit further consumption. Normal sensory systems are therefore necessary for the full enjoyment of food and are important regulators of nutrient intake. The abilities to smell and taste food are particularly important. The aroma of food can serve as a powerful appetite stimulant. After food enters the mouth, aromatic substances released from the food circulate up through the nasopharynx to the olfactory cleft where they enhance taste. The sensations of smell and taste add to the pleasure of eating while serving as chemosensory signals for food digestion by triggering salivary, gastric, pancreatic, and intestinal secretions. The texture, temperature, and quantity of food in the mouth also contribute to the hedonic qualities of a meal and serve to promote further consumption.
A significant deterioration in sight, olfactory function, taste sensation, or ability to feel the temperature and texture of food in the mouth can have a deleterious effect on eating habits and the likelihood of maintaining an adequate diet. This becomes an important concern with advanced age. Even healthy older individuals experience a modest deterioration in their ability to detect odors and to differentiate one odor from another. There is a similar pattern to the loss of taste. Compared to young people, older individuals who suffer from no diseases and take no medications have a moderately reduced ability (i.e., require greater concentrations) to both detect and to identify sweet, sour, salty, and bitter substances and amino acids such as glutamate salts. Elderly persons also have reduced ability to discriminate intensity differences and to recognize taste mixtures.
Much greater losses in taste and smell occur in association with medication usage and other health-related concerns. Tables 38-1 and 38-2 contain a representative listing of local, central nervous system, and systemic diseases; nutritional deficiencies; medications; surgical interventions; and environmental exposures (including smoking) that can cause appetite suppression and a loss of olfactory function and taste sensation. Poor oral health and many diseases that decrease mastication, salivary flow, or ability to swallow can also lead to deterioration in taste and smell and adversely affect appetite. The grinding and mixing of food with saliva play important roles in the release of volatiles and in bringing substances in contact with taste receptors, while swallowing movements are essential to pump the released volatiles up to the olfactory cleft where they are perceived.
Nutritional | Infections |
Zinc deficiency | Sinusitis |
Niacin deficiency | Acute viral hepatitis |
Vitamin B-12 deficiency | Upper respiratory tract viral infections |
Head and neck | Central nervous system |
Allergic rhinitis | Alzheimer’s disease |
Glossitis | Head trauma |
Nasal polyps | Multiple sclerosis |
Sjogren syndrome | Neoplasia |
Dental problems | Parkinsonism |
Radiation therapy | Korsakoff syndrome |
Smoking | Cranial nerve lesions |
Systemic diseases | Endocrine |
Cirrhosis of the liver | Diabetes mellitus |
Renal failure | Hypothyroidism |
Cancer | Adrenocortical insufficiency |
Panhypopituitarism | |
Iatrogenic | |
Laryngectomy | |
Chemotherapy |
Antidepressants |
Anti-inflammatories |
Antihypertensives and other cardiac medications |
Lipid-lowering drugs |
Antihistamines |
Antimicrobials |
Antineoplastics |
Bronchodilators and other asthma medications |
Muscle relaxants |
Drugs for the treatment of parkinsonism and dementia |
Anticonvulsants |
Vasodilators |
In addition to the special senses, there are various neural and humoral pathways within the gut that change with advanced age. Although controversial, some of these changes may contribute to the inability of many older individuals to adequately regulate food intake. Normally, stretch reflexes within the wall of the stomach play a key role in signaling the brain when adequate amounts of food have been ingested. Some investigators theorize that these reflexes become overly responsive in advanced age leading to early satiety and reduced food intake. Other investigations have revealed that gastric emptying is delayed with advanced age and that this may lead to slower absorption of carbohydrates, a reduction in insulin secretion, and a relative suppression of the hunger response. However, other studies have found postprandial insulin levels to be elevated in the elderly suggesting that it is the age-related change in the meal-induced pattern of insulin secretion that may be the more important determinant of food intake. There is also evidence that certain gastrointestinal hormones, such as cholecystokinin, contribute to the appetite disregulation with advancing age. Both the amount of cholecystokinin released in response to a meal and its potency in producing early satiety are greater in old compared to younger individuals. A number of other hormones have also been linked to appetite control. These include ghrelin, a gastric peptide that has apparent orexigenic properties, and leptin, a hormone produced by adipose tissue that induces satiation at high serum concentrations. Whether age- or disease-related changes in the secretion of these and other hormones are causally related to the decline in nutrient intake and loss of weight seen in many frail older adults remains controversial.
After the seventh decade of life, the importance of psychological, socioeconomic, and cultural factors to maintaining an adequate diet increases. Depression is a common, frequently unrecognized, and potentially treatable cause of a poor appetite and must always be considered when evaluating an older patient who is losing weight. A correct diagnosis is often difficult to make in the elderly, particularly when the individual suffers from other medical problems or dementia. Similarly, bereavement is associated with a lack of appetite. Poverty, lack of education, limited mobility, feeding dependency, and social isolation are also important risks. Some, though not all, studies of healthy adults have found that eating alone is associated with a lower energy intake than when eating with others and that the presence of family and close friends leads to greater nutrient intake than eating with less familiar individuals. Within institutional settings, particularly nursing homes, physical environment and ambience within the dining areas are known to affect appetite. Interventions to ameliorate the dining experience within nursing homes have been shown to improve nutrient intakes and promote weight gain. Therapeutic diets (e.g., low salt or low cholesterol) are frequently prescribed in nursing homes, often to residents who are losing weight, even though they may add little or nothing to disease management. For these reasons, the American Dietetic Association has published a position statement suggesting that use of therapeutic diets in the nursing homes be restricted.
Nutrient Requirements to Maintain Health
Daily energy requirements per kilogram of body weight generally decline with age, dropping as much as 33% between the third and ninth decade of life. Male gender and chronic disease are associated with a greater rate of decline. However, a decrease in energy requirements with age occurs even among those who remain healthy. The primary reason for this decline is the loss of muscle mass that is nearly universal with advanced age. Muscle is much more metabolically active than adipose tissue. As muscle mass is lost, the ratio of fat to lean mass increases leading to a greater drop in basal metabolic rate (BMR) than predicted by the decrease in total body mass. With advanced age, there is also a slight decline in metabolic rate per kilogram of fat-free mass. This may relate to the age-related change in the ratio of high (e.g., muscle) to low (e.g., bone) metabolic rate tissues within the fat-free mass compartment. Because the BMR generally accounts for 60% to 75% of TEE, the end result of the muscle loss is a significant decline in TEE and thus in energy requirements. It is estimated that every 10-kg loss of skeletal tissue mass results in an approximate 150 kcal/d decline in basal energy expenditure.
A second mechanism accounting for the decline in energy requirements with age is a decrease in physical activity. Energy expenditure of physical activity (EEA) generally declines with age to the same extent as BMR, accounting for approximately 15% to 35% of TEE in the majority of elderly adults. However, EEA can range from 5% of TEE in those who are bedridden to 50% in highly active, physically fit older adults. Studies of free-living older adults demonstrate that the strongest independent predictors of EEA are maximum oxygen consumption, fat-free mass, and body mass. After adjustment for body composition, there are no significant differences in TEE, BMR, or EEA between males and females. Other studies indicate that lifestyle plays a big role in determining EEA of older people, just as it does in those who are younger. Although the increased prevalence of chronic disabling disease accounts for some of the decline in physical activity with advancing age, even healthy older adults tend to be more sedentary than younger individuals.
Many chronic diseases, including congestive heart failure, Parkinson disease, and Alzheimer dementia, are associated with an increase in BMR. However, these conditions have been found to be associated with a decreased TEE because of decreased energy of physical activity. Some chronic conditions may result in an increased TEE, although this remains controversial. Individuals with Alzheimer’s disease who constantly pace would fall into this category, as would individuals with a constant tremor. Even when individuals appear to be rather sedentary, their TEE from physical activity may be greater than expected, particularly if they have certain disabilities such as neurologic disorders and amputations. These conditions sometimes result in a loss of neuromuscular energy efficiency and thus exceptional levels of energy expenditure are required to complete even basic activities of daily living.
Several different clinical methods of estimating the energy requirements of older adults have been validated with sophisticated energy expenditure measurement techniques, such as doubly labeled water studies. These estimates are reviewed in Chapter 40 and summarized in Table 40-2.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree