Nocardia
Epidemiology and risk factors
Nocardia species are a heterogeneous group of ubiquitous aerobic, gram-positive filamentous organisms that reside in soil and decaying organic matter and are associated with an array of infections in both immunocompetent and immunocompromised hosts. First described by Edmond Nocard in 1888, Nocardia infections are associated with a range of illnesses, from localized suppurative skin lesions and chronic mycetomas, to invasive pulmonary infections, bacteremia and central nervous system (CNS) infection. Although nocardiosis remains a relatively rare infection overall, with an estimated 500 to 1000 cases diagnosed annually in the United States; immunocompromised hosts, particularly those with impaired T-cell immunity, are at significant risk for infection, with prevalence rates as high as 2.0% to 3.5% in select populations. , Geographically, Nocardia infections occur throughout the world, with variations in local incidence and Nocardia subspecies attributable to differences in climate and geography. Within the United States, dry, warmer climates (such as in the Southwest) are associated with higher rates of Nocardia infection, potentially caused by increased aerosolization of pathogens with dust, or contamination of wounds with dirt. Among children, 60% to 70% of cases occur in the setting of underlying immune deficiency (e.g., systemic lupus erythematosus, solid organ transplant [SOT], bone marrow transplant (BMT), chronic granulomatous disease, or cancer), with pulmonary infection the most common manifestation, followed by central nervous system (CNS) infection, disseminated bacteremia, and skin and soft tissue disease. In contrast, approximately 30% of pediatric cases occur in otherwise immunocompetent children and typically present as lymphocutaneous disease, orbital cellulitis, arthritis, or pneumonia. Outcomes in this setting are almost uniformly good and rarely fatal. In the largest single-center study of pediatric nocardiosis to date, 31 cases of Nocardia brasiliensis infection were identified among healthy children in south Texas over a 5-year period; all presented with lymphocutaneous disease, with no noted episodes of dissemination and no reported deaths. In contrast, mortality rates among SOT and BMT recipients have been reported to be as high as 60% to 70%.
Microbiology of Nocardia
The recent advent of molecular diagnostic tools such as gene sequencing and matrix-assisted laser desorption ionization/time-of-flight mass spectrometry has led to significant changes in the classification of Nocardia species. Although classic methods of identification previously relegated most pathogenic species to a relatively limited number of Nocardia groups or complexes, ( N. asteroides complex, N. brasiliensis and N. otitidiscaviarium ), the use of molecular diagnostics has led to the discovery of more than 50 distinct pathogenic Nocardia species previously classified within these groups. As a result, the complex previously known as N. asteroides complex, once considered the most pathogenic of Nocardia complexes, has in recent years been reclassified into six distinct taxa, each of which demonstrates unique antimicrobial susceptibility patterns; these include N. nova, N. abscessus, N. transvallensis, N. brevicatena/N. paucivorans, N. cyriacigeorgica, and N. farcinica complexes. Other important pathogenic Nocardia species include N. brasiliensis, N. otitidiscaviarium, and N. pseudobrasiliensis . As a result of these classification changes, in recent years, the majority of Nocardia infections in the U.S. have been attributable to these species, specifically N. nova , N. abscessus, N. farcinica , and N. cyriacigeorgica .
Given the unique pathogenic characteristics and antimicrobial susceptibility patterns of various Nocardia species, identification of Nocardia infections to the species level is recommended. Given the recognized limits of classic phenotypic testing, current guidelines recommend the use of molecular methods to identify Nocardia isolates in cases of suspected infection. ,
Nocardiosis in solid organ transplant recipients
SOT recipients are at increased risk for nocardiosis, with high rates of associated morbidity and mortality. Although estimates vary based on the type of organ transplanted, immunosuppressive regimen, and geographic region, the frequency of Nocardia infection among kidney, heart, and lung transplant recipients has been reported to be between 0.6% and 3.5%, with incidence estimates in transplant recipients suggesting a 100- to 3000-fold greater risk for Nocardia infection than in the general population. , As can be expected from an environmental pathogen that primarily enters the host via inhalation, lung transplant recipients are likely to be uniquely susceptible to nocardiosis. Among North American SOT recipients, lung transplant recipients are at greatest risk for Nocardia infection (3.5%), followed by heart transplant recipients (2.5%) and multivisceral transplant recipients (1.3%). Infection risk among liver and kidney transplant recipients is low, with less than 1% diagnosed with nocardiosis in most studies. Potential donor-derived Nocardia transmission is another consideration in the SOT population. Although there are no published reports of donor-derived nocardiosis in pediatrics, it is listed a single time as a potential donor-derived transmission from the ad hoc Disease Transmission Advisory Committee based on the 2005-2009 Organ Procurement and Transplant Network reports. , Geography and environment are also likely to affect risk of infection among SOT recipients, with individuals residing in warm dry climates at increased risk of nocardiosis. In a study of more than 2000 SOT recipients in the American Southwest, the risk of infection was noted to be between 2 and 3 times higher across all SOT groups, compared with other regions. In this setting, lung transplant recipients remained at greatest risk (9.28%), with heart transplant (4.57%), kidney transplant (1.13%), and liver transplant recipients (0.45%) following, respectively.
Nocardia infection is associated with significant morbidity and mortality among SOT recipients, with an overall estimated 10-fold increase in 1-year mortality risk over noninfected individuals. Risk factors for infection among SOT recipients include receipt of high-dose steroids, cytomegalovirus disease in the preceding 6 months, use of tacrolimus, and high median calcineurin inhibitor levels in the preceding 30 days. , The majority of infections occur between 1 month and 1 year after, with a range of clinical presentations from predominately isolated pulmonary infections (∼70%) in North American studies to higher rates of disseminated disease (∼40%) and CNS infection (25%) in European studies.
Nocardiosis in hematologic malignancy and stem cell transplant
Hematology/oncology patients and recipients of hematopoietic stem cell transplant (HSCT) are also at increased risk for morbidity and mortality owing to nocardiosis. Incidence estimates from a systematic review between 1966 and 2004 reported 13 cases/1000 person-years among BMT recipients, 300 times greater than the estimated risk in the general population. Other more recent (2008 to 2013) retrospective studies in HSCT recipients found an incidence rate of 2.4 cases/1000 patients. Depending on the type of HSCT, such as autologous versus allogeneic, other reported incidence rates vary from 0.4% to 3.6%. Predisposing risk factors for nocardiosis in the HSCT population include high prednisone doses (≥20 mg of prednisone per day), lymphopenia, concurrent opportunistic infections (e.g., cytomegalovirus), CD4 + T cells <100 cells/μL and active graft-versus-host disease. , Sites of infection in adult HSCT and oncology patients are similar to those seen in SOT with pulmonary infection the most common (70% to 87%), followed by CNS/disseminated infection (47% to 50%), and skin (6% to 8%). Nocardia infection-related mortality in HSCT and oncology patients remains high with reports ranging from 60% to 70% mortality and one report of approximately 25% survival at 300 days after diagnosis of Nocardia.
Clinical presentation
Because of its ubiquitous presence in the environment, Nocardia often gains entry via the respiratory tract into the lungs. From there, Nocardia species can cause localized pulmonary disease or continue to spread to other sites, resulting in a diverse spectrum of infection. In general, the clinical presentation of nocardiosis tends to be similar across SOT, HSCT, and oncology patients.
Pulmonary nocardiosis is the most frequent manifestation of infection in immunocompetent and immunocompromised patients. Symptoms in both groups and across SOT, HSCT, and oncology patients most commonly consist of fever (80%) and productive cough (60%) with or without shortness of breath/dyspnea, chills, pleuritic chest pain, and/or weight loss; hemoptysis is less frequently reported (<10%). Generally, pulmonary nocardiosis presents as a subacute process with symptoms present for up to several weeks and abnormal thoracic imaging is a common finding (see “Diagnosis” section). Reports of presenting symptoms in pediatric SOT patients include fever and chest pain.
Extrapulmonary nocardiosis is common in immunocompromised patients and is often due to hematogenous dissemination to other sites or contiguous spread from a pulmonary focus into the nearby structures. Although estimates of disseminated nocardiosis vary, approximately 30% to 50% of immunocompromised patients with Nocardia infections are found to have disseminated disease, with the CNS as the most common secondary site.
The clinical presentation of patients with CNS or disseminated nocardiois has been documented in adults and can be quite variable and nonspecific. Although CNS infection can present as classic meningismus, Nocardia infections of the CNS tend to occur as one or more focal abscesses, and symptoms may also be more indolent than other more common bacterial etiologies. Clinical presentation of CNS nocardiosis covers a spectrum of symptoms from silent with no focal neurologic findings to altered mental status and unresponsiveness. Signs and symptoms at the time of clinical presentation can be those seen with any space-occupying lesion, including headaches, seizures, motor and sensory deficits, personality changes, fevers, nausea, emesis, visual changes, weight loss, and other nonspecific generalized symptoms. One case reported presenting symptoms in immunocompromised children including fever, diarrhea, lethargy, nausea, and meningismus.
Skin and soft tissue infections can occur after direct inoculation, including minor trauma, or as a manifestation of hematogenous spread. Initially, in most immunocompromised patients, it is often not readily apparent which route is responsible for the lesions seen on examination; therefore a broad workup is often necessary. Cutaneous manifestations are variable and include cellulitis, subcutaneous nodules/pustules, lymphocutaneous disease (sporotrichoid nocardiosis), abscesses, pyomyositis, and/or mycetomas. Erythema may also be present in addition to spontaneous drainage of lesions. Cutaneous nocardiosis also more frequently involves the face and lower extremities as opposed to the upper extremities or torso. Soft tissue nocardiosis as the result of disseminated infection tends to more commonly manifest as a deeper abscess or nodules rather than more superficial lesions. Localized, nondisseminated cutaneous infections are most often seen in immunocompetent patients, including otherwise healthy children.
Although localized cutaneous nocardiosis remains relatively uncommon in the SOT, HSCT, and oncology patient populations, skin and soft tissue nocardiosis is more commonly a sign of disseminated disease. In an analysis of adult SOT recipients with nocardiosis, localized skin and soft tissue infection as the only site of infection was seen in only 7% of patients, whereas skin and soft tissue infection as part of disseminated infection was seen in 32%.
Other less common manifestations include bone or joint, endocarditis/pericarditis, renal, and ocular infections. Although Nocardia bacteremia is generally regarded as a relatively uncommon finding, it has been reported in approximately 8% of adult SOT and 27% of adult HSCT recipients with nocardiosis. , Central venous catheter (CVC)-associated Nocardia has been reported in immunocompromised pediatric and adult patients, either as disseminated (or secondary) bacteremia or as central line–associated bloodstream infection alone. Symptoms include fever, chills, malaise, pain, and/or erythema at the CVC insertion site.
Prevention and prophylaxis
There is no proven intervention that has been shown to be clearly effective in preventing nocardiosis in SOT, HSCT, or oncology patients. Despite what would appear to be an appealing biologically plausible link, and historic reports on the benefit of trimethoprim-sulfamethoxazole (TMP/SMX) prophylaxis, evidence of clear efficacy of TMP/SMX prophylaxis for nocardiosis remains elusive. One case-control study in adult SOT recipients with nocardiosis reported up to 18% of cases occurring in the setting of TMP/SMX prophylaxis given for Pneumocystis jirovecii pneumonia (PJP), and although there was a slight reduction in risk (odds ratio 0.36, 95% confidence interval 0.14 to 0.93, P = 0.03), this was not present in multivariable analysis. Other studies in immunocompromised patients have reported similar, and even greater, rates of breakthrough nocardiosis—up to 69% in some cases. Of the Nocardia isolates from those patients with breakthrough infection treated with TMP/SMX, the majority of isolates (79% to 100%) are typically found to be susceptible to TMP/SMX, leaving resistance to TMP/SMX as an unlikely explanation for the failure.
The most commonly proposed explanation for this somewhat confounding result is that the efficacy of TMP/SMX for the prevention of nocardiosis may be dependent on dose and frequency, and routine prophylaxis (often given 2 to 3 times per week for PJP) is not sufficient. Data are similar in the HSCT population in that TMP/SMX does not reliably prevent nocardiosis. Although some report a potential benefit of TMP/SMX prophylaxis in preventing disseminated nocardiosis, this is also not a reproducible finding. Additionally, there are reports of breakthrough nocardiosis in HSCT recipients receiving daily (single-strength) TMP/SMX. While the data on efficacy of TMP/SMX for prevention of nocardiosis remain mixed, fortunately there is little evidence that prophylaxis given for other reasons (i.e., PJP or toxoplasmosis) will select for TMP/SMX-resistant Nocardia isolates.
Diagnosis
The diagnosis of Nocardia infection typically relies on culture and identification of the organism from the appropriate clinical site. Because of the broad variability in clinical manifestations of nocardiosis as well as the other potential pathogens on the differential for most immunocompromised patients, the time to definitive diagnosis of Nocardia can be prolonged, with interval from symptom development to diagnosis of 20 to 30 days in both SOT and HSCT. Rarely, patients in both groups can have symptoms for more than 3 months before diagnosis. Culture of Nocardia species requires special consideration, as opposed to simply obtaining routine aerobic and anaerobic bacterial cultures. Because of their relatively slow growth, isolation of Nocardia can require extended incubation periods. In some cases, Nocardia species may grow after a minimum of 2 to 5 days, whereas in other cases up to 4 weeks can be necessary; therefore the microbiology laboratory should be made aware of the clinical suspicion for Nocardia and samples should also be set up on media optimized for fungi and/or mycobacteria to allow for longer incubation times. Although standard fungal and mycobacterial cultures readily grow Nocardia , in some cases the digestion and decontamination procedures used for mycobacterial culture may render Nocardia nonviable. Therefore mycobacterial culture media should not be the sole method used for testing, and culture for fungi may be preferred.
Direct examination of gram-stained specimens can demonstrate filamentous, beaded, and/or branching rods that stain weakly gram-positive and are partially acid-fast. In the appropriate clinical setting, microscopic visualization of the organism can allow for an early, presumptive diagnosis while complete culture results are still in process.
In clinical practice, the approach to diagnosing nocardiosis often requires a combination of clinical suspicion, examination findings, and radiographic studies, which in turn leads to site selection for sampling of material for culture (i.e., blood, spinal fluid, respiratory fluid, and/or biopsy specimen). See Fig. 30.1 for a proposed diagnostic workup schema for Nocardia ; other/additional diagnostic studies may be indicated to evaluate alternative etiologies and/or disseminated nocardiosis to less common sites, such as musculoskeletal, ocular, cardiac, or other sites. As with many infections in immunocompromised hosts, a low threshold for broad diagnostic testing and imaging is often warranted.
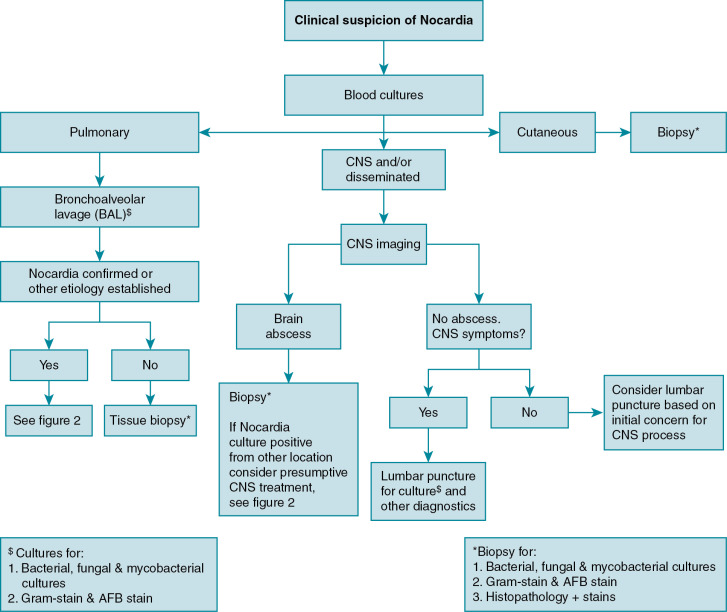
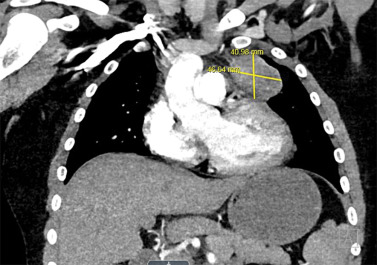
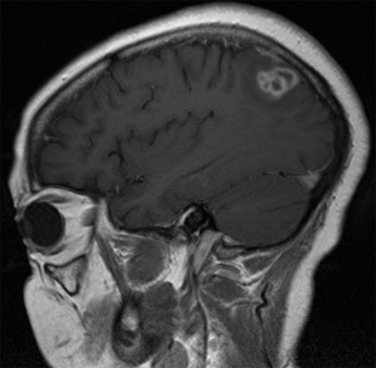
Although some findings indicative of pulmonary nocardiosis can be seen on a chest radiograph, computed tomography (CT) is preferred to evaluate and characterize any lesions ( Fig. 30.2 ). Common findings in SOT recipients include airspace consolidation (64%), nodules (57%), masses (21%), pleural effusions (28%), and mediastinal/hilar lymphadenopathy (15%). Nodules can be variable in distribution and appearance, with either ill-defined or well-defined borders, smooth or spiculate margins; lesions may be solitary or in clusters and may also be cavitary (40%). In one series of adult SOT recipients, 43% of those ultimately found to have CNS nocardiosis had no neurologic abnormalities on examination. Therefore, CNS imaging should be performed in any immunocompromised host with nocardiosis, once the diagnoses is established. Contrast-enhanced magnetic resonance imaging is the modality of choice, as CT imaging may not be sensitive enough to detect subtle findings and small lesions. Imaging in CNS nocardiosis is variable, with up to 80% of imaging in SOT recipients showing multiple lesions made up of a combination of 53% bihemispheric, 93% supratentorial, and 30% infratentorial locations. Ring-enhancing lesions, sometimes with surrounding edema ( Fig. 30.3 ), in an immunocompromised patient should place Nocardia firmly in the differential diagnosis in addition to other etiologies, such as toxoplasmosis.