New insulin developments
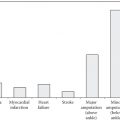
Insulin has traditionally been considered a treatment of last resort for individuals with type 2 diabetes, delayed until all other efforts by the patient and healthcare provider have failed. As β-cell function declines over time, the need for replacement insulin will increase in order to normalise blood glucose levels. β-cell decline can occur due to a number of factors, and the rate of β-cell decline and the degree of insulin resistance will be different for each individual. As a result, the right time to begin insulin will differ from person to person. In recognition of this, recent treatment guidelines recommend the use of insulin, in particular basal insulin, as part of a treatment regimen earlier in the disease process.
Insulin should not be seen as the last resort in optimising glycaemic control or as failure by the patient to control their diabetes, but rather as an option to optimise blood glucose control to prevent longer term complications. As many people are reluctant to commence multiple daily injections, insulin initiation often involves basal-only therapy in conjunction with existing oral anti-diabetes medications. Advantages of this approach include reduced risk of weight gain, reduced risk of hypoglycaemia, a simpler treatment regimen and better blood glucose control during insulin initiation and dose adjustments. Thereafter, insulin regimens that are more tailored to individual needs are likely to result in greater acceptance and patient adherence. To meet this need, a range of new insulins and insulin combinations have been developed that aim to prolong the duration of action of basal insulins; shorten the time to peak action of rapid-acting insulins; reduce peak variability, hypoglycaemia and weight gain; and provide greater flexibility in dosing time from day to day.
BASAL INSULINS
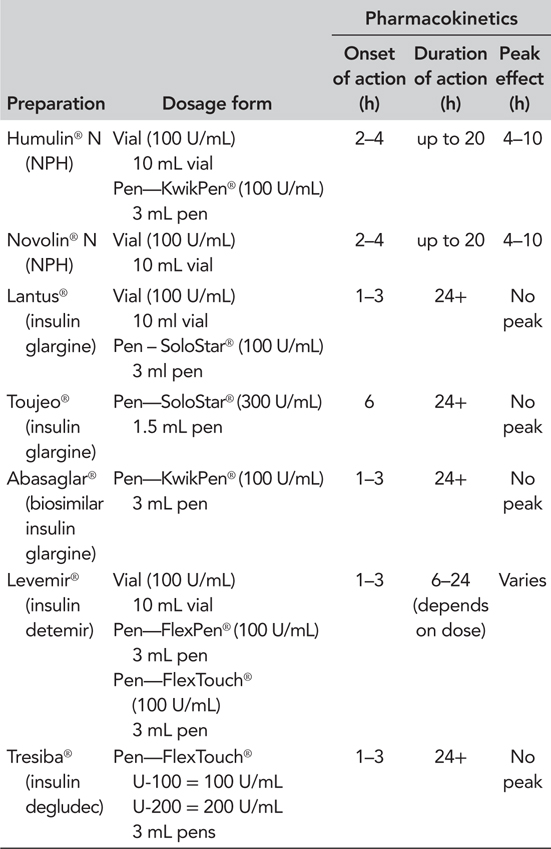
When initiating insulin therapy in a patient who is already on oral anti-diabetes medication, a basal (long- or intermediate-acting) insulin can be chosen to improve nocturnal and fasting blood glucose. Combining a glucagon-like peptide 1 receptor agonist (GLP-1 RA) with basal insulin is also an effective regimen for improving glycaemic control without increasing the risk of hypoglycaemia or weight gain (Vora et al., 2013). The basal insulin options of insulin neutral protamine hagedorn (NPH), glargine and detemir, have recently been joined by an insulin glargine biosimilar and the new ultra-long-acting basal insulins, insulin degludec and glargine U300 (Table 5.1).
Biosimilar insulins
Biosimilar insulins are the insulin equivalent of lower cost generic drugs, and several are in development for launch after the patents on analogue insulins expire. In contrast to drugs with relatively simple chemical structures, where generic copies can be produced by chemical synthesis, the complex structure of human insulin and its analogues means that similar versions can only be manufactured by a biotechnological approach using genetically modified bacteria and yeast. The biological conditions in which the insulin is manufactured, such as the incubation conditions in different laboratories using slightly different strains of bacteria and yeast, will lead to the production of similar, but not identical insulins (Heinemann, 2012).
The first such insulin to become available is biosimilar insulin glargine. This has demonstrated similar pharmacodynamics and pharmacokinetics and a similar safety and efficacy profile to insulin glargine (Rosenstock et al., 2015b). The National Institute for Health and Care Excellence (NICE) position statement on biosimilars states that NICE guidance on a product also applies to relevant licensed biosimilar products, which subsequently appear on the market. However, in the first instance, it may be appropriate to limit biosimilar insulin treatment to new patients or patients assessed as needing a medication change until clinical experience of the treatment and its delivery device has been gained. Insulin glargine and biosimilar insulin glargine use slightly different delivery devices.
Insulin degludec
Insulin degludec has been extensively studied in the BEGIN®
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree