New Approaches: Robotics and Endoscopic Head and Neck Surgery
F. Christopher Holsinger
INTRODUCTION
Minimally invasive and endoscopic head and neck surgery1 (eHNS) represents the natural evolution of the surgical disciplines within the multidisciplinary treatment paradigm. Yet, for years, head and neck surgery was virtually synonymous with “radical” transfacial and/or transcervical procedures. Although there were “conservation” or organ-preservation procedures in the larynx, such as the vertical partial laryngectomy or the open supraglottic laryngectomy, (Chapter 18), operative indications were often too narrow for surgery to play a significant role in treatment. Thus, multidisciplinary care for head and neck cancers gradually shifted toward a radiation-based approach. Multidisciplinary care was virtually synonymous with nonsurgical therapy.
However, an evolution in our understanding of transoral anatomy, the advent of computer-assisted surgery, and a host of new technologies has ushered in a new era for the head and neck surgeon. For tumors of the upper aerodigestive tract, especially the larynx and oropharynx, eHNS has naturally developed around transoral exposure, using either transoral laser microsurgery (TLM) or transoral robotic surgery (TORS) or a combination of the two (Table 6.1). With greater understanding of these minimally invasive approaches, surgeons have now begun to apply eHNS techniques to the neck systematically. Finally, using transaxillary and retroauricular approaches, surgeons have demonstrated the feasibility of operating on the neck for both thyroid and parathyroid surgery and even lymphadenectomy through small cervical incisions with video-assistance or through small inconspicuous incisions located some distance from the primary tumor site, hidden in the folds of the axillary or behind the ear.
Here, we review these developments, focusing on rationale, anatomy, and technical aspects, as well as indications and limitations of minimally invasive and eHNS. The term “eHNS” is preferred, since every approach described below from robotic surgery to TLM or minimally invasive video-assisted thyroid surgery (MIVAT) utilize an endoscope to provide unparalleled access and visualization of head and neck surgical anatomy. More importantly, eHNS is independent of a particular technology or medical device industry, and focuses on surgical technique and its evolution, unfettered by the bonds of commercial affiliations and sponsorship.
TABLE 6.1 Endoscopic Head and Neck Surgery | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||
TRANSORAL LASER MICROSURGERY
Laser Physics and Choice of Technology
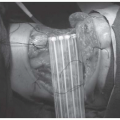
eHNS was born in 1972 within the field of laryngology using transoral exposure for the resection of benign and early limited cancers by Strong and Jako.2 Since then, the technique of TLM has emerged as a standard of care for early laryngeal cancer (Chapter 18b) and also an important surgical approach for selected tumors of the oropharynx, supraglottis, and hypopharynx. TLM is performed via direct laryngoscopy, with an operating microscope, microsurgical instruments, and the surgical carbon dioxide (CO2) (Fig. 6-1), pulsed potassium titanyl phosphate (KTP), or thulium laser.
Since a surgical laser serves as a scalpel, the surgeon must understand this tool and have a firm grasp of laser physics and the thermal and mechanical aspect unique to a particular laser’s soft-tissue interactions. In TLM, the laser is used to cut, not to ablate or to simply cauterize tumors, delivering energy in the form of light directly to target tissue. Light energy is absorbed directly without mechanical or electrical trauma, but when absorbed by soft tissue is transformed into heat, producing a photothermolysis. As more laser energy is absorbed by a particular
tissue, the laser’s chromophore, covalent bonds are released and tissue is vaporized, usually at the boiling point. The physics of the CO2 laser make it ideally suited for head and neck surgical applications. Water, as the primary component of most cells in the human body, serves as an efficient chromophore for light energy from the CO2 laser, due to its frequency of 10,600 nm. As the cells absorb the light energy from the laser, the cells’ temperature rises quickly and at 100°C the cells vaporize. This soft-tissue interaction allows the surgeon to cut and coagulate tissue without electrocautery, thus minimizing collateral damage to adjacent neurovascular, mucosal, and muscular structures.
tissue, the laser’s chromophore, covalent bonds are released and tissue is vaporized, usually at the boiling point. The physics of the CO2 laser make it ideally suited for head and neck surgical applications. Water, as the primary component of most cells in the human body, serves as an efficient chromophore for light energy from the CO2 laser, due to its frequency of 10,600 nm. As the cells absorb the light energy from the laser, the cells’ temperature rises quickly and at 100°C the cells vaporize. This soft-tissue interaction allows the surgeon to cut and coagulate tissue without electrocautery, thus minimizing collateral damage to adjacent neurovascular, mucosal, and muscular structures.
If laser beam is not efficiently delivered, tissues are burned, not completely vaporized. Inefficiently denatured protein appears to the surgeon as carbon debris, or “char,” which further interferes with the surgeon’s visualization and obstructs the efficient delivery of laser energy to the target tissue resulting in greater subablative heat build-up. Using a pulsed laser is an important way to avoid thermal injury: that is, turning the laser on and off in microsecond bursts. In this pulsed mode, thermal relaxation time is reduced and target tissues can dissipate heat before char develops. Also, thermal injury can be diminished by reducing exposure time. That is, using a “repeat-delay” setting, a pulsed laser can be turned on every 0.1 to 0.3 seconds, further reducing thermal damage during TLM.
In addition to these general considerations, the long wavelength of the CO2 laser carries with it some limitations. The beam itself is invisible. Thus, a visible and parallel beam of light from a Helium-Neon laser (wavelength of 656 nm) is used to identify the invisible 250 µm “spot” generated by the CO2 laser. A mirror micromanipulator is connected to a microscope, and the laser beam can then be directed with a small surgical joystick. For many patients with tumors throughout the laryngopharynx, direct “line-of-sight” exposure is achieved, whether by direct transoral exposure to the oral cavity, tonsil, and palate or via endoscope for the deeper oropharynx, larynx, and hypopharynx. However, this line of sight restriction may limit the “working space” of the laser within which the surgeon can work and proves problematic as tumour size increases or patient anatomy restricts line-of-sight delivery. The Omni Guide System (Omni Guide, Inc., Cambridge, Massachusetts) introduced the first widely commercially available fiber-optic CO2 laser.3 With a fiber, the surgeon can wield and direct the fiber-optic laser almost as a scalpel. In so doing, the CO2 laser can be used “around-corners” and into the difficult recesses of the head and neck, thus overcoming some of the limitations associated with line-of-sight exposure.4 The flexibility of the CO2 laser fiber delivery system permits either hand-held operation or even mounting to a specialized robotic manipulator. Yet, these hollow-core fibers can be fragile and fail when handled too aggressively or bent at extreme angles. Other teams are at work to deliver CO2 laser energy through glass fibers that might provide greater durability.5
Further technical refinement for the CO2 laser was first presented by Remacle et al.6 with the introduction of the scanning robotic micromanipulator. The surgeon can use this robotically crafted, pulsed laser beam in several different shapes and spot sizes ranging from 0.4 to 5 mm, using a computer-assisted algorithm. These settings allow the surgeon a great deal of precision to customize the laser-soft tissue interaction, resulting in greater finesse, especially for instance when operating on lesions of the true vocal cord. Furthermore, the surgeon can choose from several patterns or cutting shapes, instead of just a 250 µm spot. Using this “digital acublade” on varying pulsed modes, Remacle et al.6 showed significant reduction in thermal injury zone to 12.5 µm and increased operative precision, when compared to the traditional “dot-by-dot” joystick micromanipulator approach.
Finally, thulium-based fiber-optic lasers7 have similar properties to CO2 lasers and demonstrate promise for benign laryngeal and tracheal disease, as well as, for cancer. The 2,013-nm wave length of this laser allows its energy to be delivered by a small caliber glass fiber, yet it retains water as a chromophore. This laser appears to offer a smooth vaporization pattern and excellent hemostasis, similar to that realized with the pulsed KTP laser,8 but with less risk of deep tissue penetration.
Technique and Its Role in the Multidisciplinary Paradigm
Independent of the novel and emerging technologies in TLM, head and neck surgeons now routinely employ this transoral endoscopic approach for tumors of the upper aerodigestive tract. Traditionally, these techniques fell under the purview of laryngologists. Steiner and Ambrosch9 first paved the way for broader use and greater acceptance of TLM through head and neck oncologic surgery. First and foremost, the laser is used as a precise endoscopic scalpel, to cut and to coagulate, not to ablate or cauterize. Furthermore, TLM, as espoused by Steiner and Ambrosch, challenged widely held belief in Halsted’s fundamental surgical principle of en bloc resection.10 With TLM, as a tumor is transected, precise visualization under the operating microscope reveals the full depth of tumor. By understanding this third dimension of the resection, the surgeon can optimize margin of resection. Oncologic outcomes from TLM are discussed in further detail in Chapter 18b, but by conserving the maximum amount of normal mucosa, crucial structures are preserved and function is optimized. Preservation of laryngopharyngeal function is accomplished through maintenance of at least one valve of the larynx for airway protection, swallowing, and native voice.11 In fact, the promise of TLM and endoscopic head and neck surgical approaches to larynx cancer and that of other sites within the upper aerodigestive tract is this: patients who undergo these procedures, especially for T1-2 tumors, tend to fare remarkably better than was expected when these techniques were first introduced. Transoral endoscopic resection preserves the laryngeal
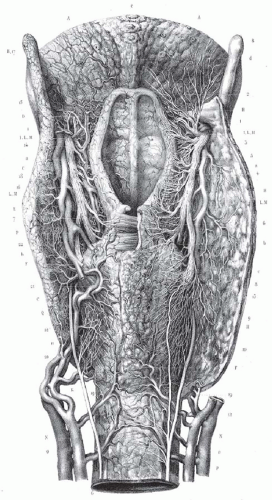
cartilaginous and musculature framework in the neck. The endterminal branches of the superior laryngeal nerve (Fig. 6-2) are preserved providing rapid return of laryngopharyngeal sensation and thus swallowing function. In an unradiated patient, the repeated and constant use and soft-tissue remodeling appears to restore anatomy to its near normal state, with a surprising degree of associated normal function. For instance, a surgical defect for a T1 verrucuous carcinoma, that requires a deep resection of the mucosa and even a portion of the vocal ligament, heals over time without significant deformity and good vocal outcomes (Fig. 6-3). For larger tumors of the larynx, with a more complex defect, from glottic larynx through the ventricle and into the false cord, the same postoperative healing appears to restore normal function (Fig. 6-4). Ultimately, the human body’s remarkable restorative capacity lies at the heart of the opportunity and potential for eHNS within the multidisciplinary paradigm.
cartilaginous and musculature framework in the neck. The endterminal branches of the superior laryngeal nerve (Fig. 6-2) are preserved providing rapid return of laryngopharyngeal sensation and thus swallowing function. In an unradiated patient, the repeated and constant use and soft-tissue remodeling appears to restore anatomy to its near normal state, with a surprising degree of associated normal function. For instance, a surgical defect for a T1 verrucuous carcinoma, that requires a deep resection of the mucosa and even a portion of the vocal ligament, heals over time without significant deformity and good vocal outcomes (Fig. 6-3). For larger tumors of the larynx, with a more complex defect, from glottic larynx through the ventricle and into the false cord, the same postoperative healing appears to restore normal function (Fig. 6-4). Ultimately, the human body’s remarkable restorative capacity lies at the heart of the opportunity and potential for eHNS within the multidisciplinary paradigm.
TRANSORAL ROBOTIC APPROACHES
Robotic Technology and Its Application in eHNS
Although the term “robot” dates back to a 1921 play Rossum’s Universal Robot12 by a Czech playwright,12 the widespread use of robotics in surgery has debuted only in the last decade. In 2007, “robotic surgery” was defined as any “surgical procedure or technology that adds a computer technology-enhanced device to the interaction between a surgeon and a patient during a surgical operation and assumes some degree of control heretofore completely reserved for the surgeon.”13
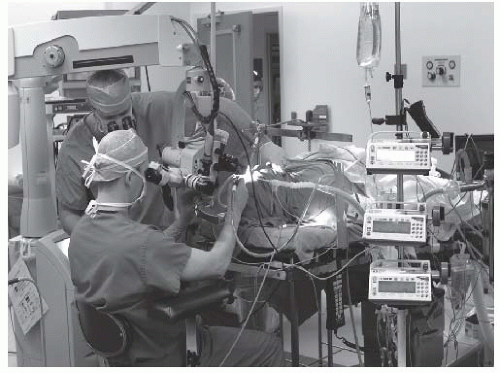
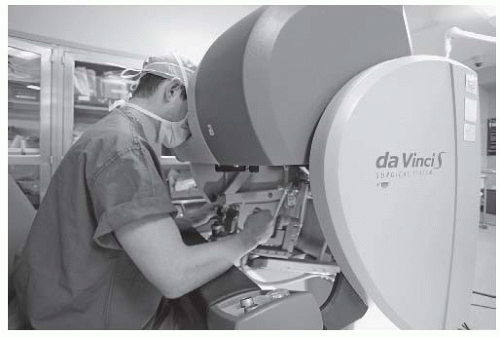
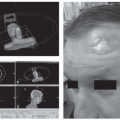
At present, there is only a single commercially available surgical robot. While the da Vinci Surgical System (Intuitive Surgical Inc., Sunnyvale, CA) is widely used to facilitate laparoscopic minimally invasive surgery in urological, abdominopelvic, thoracic, and cardiac surgery, its use for “transoral otolaryngology” procedures was approved by the FDA in December 2009.14 The da Vinci is not a singular “robot” but rather a surgical system in three parts or, to be more precise, three surgical carts. At the operating room table, surgical instruments are deployed from a “patient-side” cart and are safely placed by the surgeon within the patient’s body under direct and/or endoscopic guidance. A binocular 8.5- or 12-mm endoscope with dual 0- or 30-degree optics is used to visualize target anatomy. The three-dimensional surgical anatomy is then recreated using the “vision” cart, where computer processing links the camera’s image and the real-world spatial relationships of the instruments in a virtual environment. The surgeon, sitting at a remote console cart (Fig. 6-5) can then operate within this virtual three-dimensional environment using “master-controllers” (Fig. 6-6) that control and direct movements of the robotic instruments. These master controllers bear a remarkable similarity to joysticks and other hand-held devices employed in video games and other virtual reality simulators. The surgeon has an unprecedented perspective on the surgical anatomy as well as the ability to operate with 540 degree of wristed instrumentation and at either 0- or 30-degree angle. Furthermore, motion scaling increases precision and reduces the larger hand movements required by humans while eliminating tremor and fatigue. This surgical system permits the surgeon to interact with surgical anatomy with a novel perspective, around corners, in otherwise accessable places, and in ways that would not otherwise be possible. In other words, robotic surgery represents a fundamental advance in the art and science of surgery. For the first time, the surgeon and his hands are physically separate from the patient (Fig. 6-7). Real surgery is performed in a virtual environment. With further technological refinement and innovation, as stereoscopic cameras and instruments become smaller and more miniature, the limitations in what can be achieved will become even fewer.
The first transoral robotic head and neck procedure was performed by McLeod and Melder15 in 2003 at the Walter Reed Army Medical Center. Using a slotted and custom-modified Lindholm laryngoscope, the authors resected a vallecular cyst with the first-generation “standard” da Vinci robotic system. Their report was finally published in 2005; that same year, Hockstein et al. first demonstrated the feasibility of transoral robotics for more extensive laryngopharyngeal surgery. “Hockstein’s leap” was to place robotic arms through oral and oropharyngeal retractors, rather than traditional laryngoscopes, to more reliably facilitate surgery and thus significantly reduce external collisions.16,17 Using standard tubed laryngoscopes, this team documented limited mobility of the robotic instruments (either 8 or
5 mm) as well as the stereo-endoscopic camera. In fact, using this configuration, movement of the robotic arms tended to move the laryngoscope significantly, which suddenly and unpredictably changed visualization—a limitation that precluded safe simulated surgical procedures, even in a mannequin model. However, when the McIvor oropharyngoscope was utilized, instrument collisions were reduced or eliminated entirely in the oropharynx and was still possibly in both the hypopharynx and supraglottis. Difficulty in accessing the glottic larynx persisted using the da Vinci System.
5 mm) as well as the stereo-endoscopic camera. In fact, using this configuration, movement of the robotic arms tended to move the laryngoscope significantly, which suddenly and unpredictably changed visualization—a limitation that precluded safe simulated surgical procedures, even in a mannequin model. However, when the McIvor oropharyngoscope was utilized, instrument collisions were reduced or eliminated entirely in the oropharynx and was still possibly in both the hypopharynx and supraglottis. Difficulty in accessing the glottic larynx persisted using the da Vinci System.
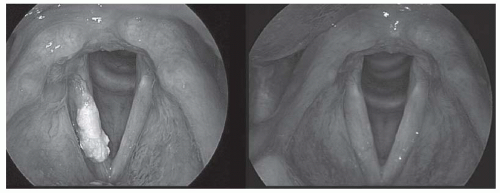 FIGURE 6-3. Preoperative (left) and postoperative (right) views of a T1 verrucous carcinoma resected via transoral laser microsurgery. The surgical defect is not evident, despite submucosal resection down to the vocal ligament. Adaptive remodeling of the larynx after transoral laser microsurgery may provide better functional outcomes that expected, when the proper lesion is choice. No radiation therapy was given. (See color insert). |
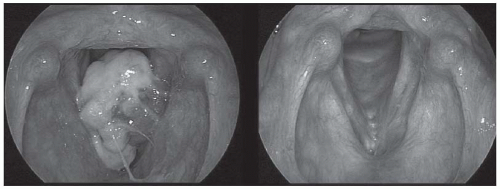 FIGURE 6-4. Preoperative (left) and 3-year postoperative (right) views of a squamous carcinoma of the larynx. CT imaging staged this as a T3 lesion, though the tumor was pedunculated from the undersurface of the left false cord. The tumor was resected via transoral laser microsurgery and no postoperative radiation therapy was given. (See color insert.) |
 FIGURE 6-6. The surgeon uses “master controllers” which control and direct movements of the robotic instruments which have been placed within the patient. |
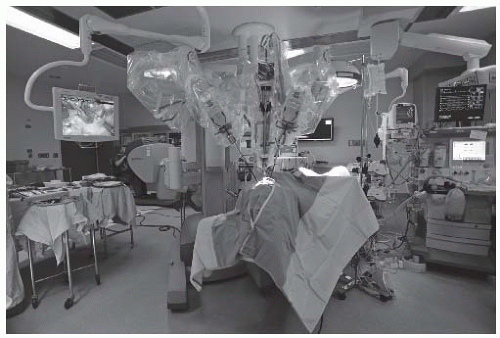 FIGURE 6-7. This intraoperative photograph demonstrates the relationships between the patient (foreground; covered in drapes), the patient-side robotic surgical cart, delivering the instruments into the oropharynx, and the remote surgical console (back left) where the surgeon operates. Robotic surgery incorporates real surgical maneuvers performed in a virtual environment.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|