Introduction
- ■
Neutrophils, eosinophils, and basophils are a subset of white blood cells characterized by the presence of granules and collectively referred to as granulocytes. Granulocytopenia is a decrease in the absolute count of these three cell lines while neutropenia is a decrease in only the absolute neutrophil count (ANC). However, for practical purposes the terms granulocytopenia and neutropenia are often used interchangeably.
- ■
It is important to understand normal physiology in order to understand neutropenia and its complications. Most neutrophils reside in the bone marrow in mitotic (myeloblasts, promyelocytes, myelocytes) and postmitotic (metamyelocytes, bands, and then neutrophils) stages. Of the neutrophils in the circulation, an equal proportion make up the marginal and the nonmarginal pool. From the circulation, neutrophils enter tissue as a result of proinflammatory trafficking cytokines. Neutropenia is a reduction in the nonmarginal pool of neutrophils which constitute only 4% to 5% of total neutrophil stores.
Neutropenia is defined as an absolute neutrophil count (ANC) of less than 1500 cells/µL; ANC of less than 1000 cells/µL is considered moderate neutropenia and ANC of less than 500 cells/µL is considered severe neutropenia. The risk of infection is greatest with severe neutropenia.
Etiology of Neutropenia
- ■
Neutropenia is a common complication seen in various malignant conditions. There are many contributing factors that should be considered in patients with malignancy. Table 2.1 describes causes of neutropenia commonly seen in patients with malignancy. Although neutropenia is the most common dose limiting toxicity (DLT) of chemotherapy, it is important to consider the differential diagnosis to determine the appropriate management for each patient.
TABLE 2.1
Causes of Neutropenia in Cancer Patients
Causes of Neutropenia in Malignancy
Mechanism
Chemotherapy ( the chemotherapy regimen remains the strongest determinant in the likelihood of developing neutropenia )
Myelosuppressive effects due to cytotoxicity
Chronic lymphoproliferative disorders – natural killer cell lymphomas (large granular lymphocytic leukemia), hairy cell leukemia, and chronic lymphocytic leukemia (CLL)
Bone marrow infiltration
Radiation
Cytotoxic Effects
Autoimmune conditions: Systemic lupus erythematosus (SLE), aplastic anemia, Crohn’s disease
Presence of antineutrophil antibodies
Rheumatoid arthritis (Felty’s syndrome)
Hypersplenism
Granulomatous infections
Bone marrow infiltration
Viral infections (e.g., CMV, EBV, HIV)
Bone marrow suppression
Parasitic infections (e.g., malaria)
Hypersplenism, multifactorial
Bacterial infections (e.g., typhoid, tuberculosis)
Multifactorial
Hemophagocytic lymphohistiocytosis (HLH)
Infiltration of bone marrow, hypersplenism
Antibiotic-induced isolated neutropenia (e.g., quinidine, hydralazine, β-lactams)
Maturation arrest of myeloid lineage
Benign ethnic neutropenia (BEN; also known as constitutional neutropenia)
Inherited predisposition
Cyclic neutropenia (21-day cycling of neutrophils)
Inherited (autosomal dominant, ELA2 gene) and acquired causes
CMV, Cytomegalovirus; EBV, Epstein-Barr virus.
- ■
There are several ways in which chemotherapy predisposes to infections. While neutropenia is an important etiology, chemotherapy can also impair physical barriers created by mucosal surfaces, the first line of defense against infections. Classic signs of inflammation including dolor (pain), calor (heat), rubor (redness), tumor (swelling), and functio laesa (loss of function) may be dampened in such patients due to blunting of the function of neutrophils. Thus increasing the chance of infections to be missed. Fever is usually the only sign of infection in neutropenic infections and thus warrants special vigilance.
Risk Assessment of Adults With Chemotherapy-Induced Neutropenia
- ■
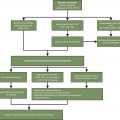
It is helpful to stratify patients into different risk categories for effective management. This risk assessment should be conducted prior to the first cycle of chemotherapy and revisited with subsequent cycles as needed. Although all patients with febrile neutropenia require antibiotics, the route of antibiotic therapy (oral vs. intravenous), the setting (outpatient vs. inpatient), and the duration of such therapy is decided after assessing the risk of febrile neutropenia. The decision about whether to give prophylactic granulocyte colony stimulating factor (G-CSF) is also made after assessing the risk of febrile neutropenia. Such a risk may be affected by patient, disease, or treatment-related factors ( Table 2.2 ).
TABLE 2.2
Risk Factors Associated with Development of Neutropenia
Patient-Related Risk Factors
Disease-Related Risk Factors
Treatment-Related Risk Factors
Age (>65 years)
Advanced stage of malignancy
Time since last chemotherapy (<7 days)
Gender (female)
Lymphopenia ,
High-dose chemotherapy ,
High body surface area
Bone marrow involvement by malignancy
Higher number of planned cycles
Comorbidities
Raised lactate dehydrogenase level in lymphomas
Colony-stimulating factor prophylaxis (inverse relationship)
Nutritional status (malnourishment, including serum albumin ≤3.5 mg/dL) ,
Absolute neutrophil count (inverse relationship)
Low baseline and first-cycle nadir blood counts
Poor performance status
C-reactive protein concentration >15 mg/dL
Longer hospital stay >10 days
Hematological malignancy as compared with solid organ malignancies
- ■
The importance of neutropenia cannot be understated. One of the feared complications of chemotherapy-induced neutropenia is febrile neutropenia which predisposes to high risk infections with high mortality. There are different universally followed risk assessment criteria for stratifying patients into low- and high-risk categories. The level of risk thus implies risk of serious complications, including prolonged hospitalizations and death in patients with neutropenic fever.
- ■
The Multinational Association for Supportive Care in Cancer (MASCC) Score
The MASCC score is an internationally validated score to stratify patients by risk. A MASCC risk-index score ≥21 identifies low-risk patients with a positive predictive value of 91%, a specificity of 68%, and a sensitivity of 71%. This risk score is incorporated into the National Comprehensive Cancer Network (NCCN) guidelines for management of patients with neutropenic fever.
MASCC uses the following criteria to calculate a score :
- ■
Burden of illness
- ■
No or mild symptoms (5 points)
- ■
Moderate symptoms (3 points)
- ■
Severe symptoms (0 points)
- ■
- ■
Comorbidities
- ■
No hypotension (systolic blood pressure >90 mmHg) (5 points)
- ■
No chronic obstructive pulmonary disease (4 points)
- ■
Solid tumor or hematologic malignancy with no history of previous fungal infections (4 points)
- ■
No dehydration requiring parenteral fluids (3 points)
- ■
- ■
Status
- ■
Outpatient status at the time of onset of the neutropenic fever syndrome (3 points)
- ■
- ■
Age
- ■
Lesser than 60 years (2 points)
- ■
≥ 60 years (0 points)
- ■
- ■
Depending on the total score, patients can be stratified into low (21–26 points) or high-risk (0–20 points) categories. Patients with a low MASCC score may be treated in the outpatient setting with oral antibiotics. On the other hand, patients with a score of less than 21 are at high risk of infections and complications and should be treated in the inpatient setting with broad spectrum intravenous antibiotics. Although the MASCC score is widely used, it has some potential limitations. First, it may not be applicable to more stable patients in the outpatient setting. Second, the duration of neutropenia, an important criteria in the NCCN risk assessment, is not incorporated into the MASCC score.
NCCN risk assessment
- ■
A more commonly used approach is the NCCN risk assessment, which is described in Table 2.3 . The NCCN assessment categorizes patients into low risk, intermediate risk, and high risk based on clinical features. In general, low-risk patients can be treated in the outpatient setting with oral antibiotics and high-risk patients should be treated in the inpatient setting with IV antibiotics. Intermediate-risk patients should receive individualized assessments regarding therapy.
- ■