INTRODUCTION
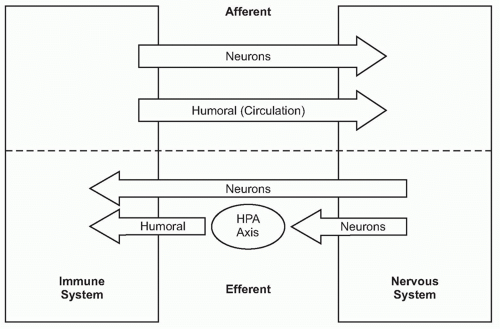
A fundamental principle in biology is that organ function is maintained within a narrow physiologic range, one optimal for health. Heart rate, body temperature, and the output of all organ systems are controlled by homeostatic mechanisms. The nervous system occupies a crucial role in establishing this healthy range of organ function. There is continuous communication between the nervous system and the other organ systems, linked by two major information highways
(Fig. 35.1). One route is neural, which transmits action potentials via nerves that travel directly into the organs back and forth to the nervous system. The other is humoral, which utilizes the circulatory system to deliver hormones, inflammatory mediators, and other soluble factors that shuttle information from the immune system to the nervous system, and back again from the neuroendocrine organs to the immune system.
The immune system, the principle organ system of host defense, exerts significant influences on the function of the other organ systems, and the magnitude of these responses is crucial to physiologic homeostasis. For example, cytokines modulate the metabolism of hepatocytes, adipocytes, and skeletal muscle cells to mobilize fatty acids, glucose, and other energy stores required for new protein synthesis in lymphocytes and other immune cells to support antibody production, and other essential molecules. Inflammatory mediators also modify the activity of cells in the nervous system, disrupting homeostasis and producing fever, anorexia, and avoidance behavior. These protective responses, which together improve the odds of the organism surviving infection and injury, are frequently the major signs and symptoms of disease. Decades of prior work revealed how the immune system communicates with the nervous system by utilizing both communication routes, the humoral and neural, to change the behavior of the organisms. Quite recently, however, advances in neurophysiology and immunology elucidated mechanisms by which these incoming signals from the immune system stimulate regulatory signals that return from the nervous system to the immune system through neural networks that maintain immunologic homeostasis.
These neural reflexes are essential for maintaining a healthy immune response for optimal function during infection and injury, termed “immunologic homeostasis.” Impairment of these fundamental reflex mechanisms leads to unregulated immune responses, uncontrolled cytokine production, and deviations of organ system function outside of the homeostatic range. This chapter focuses on the basic principles underlying the neurophysiologic mechanisms that regulate immunologic homeostasis.
ORGANIZATION OF THE NERVOUS SYSTEM
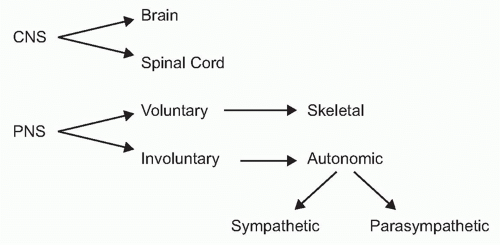
To understand the role of neural reflexes and the neuroendocrine system in maintaining immunologic homeostasis, it may be illustrative to review the basic organization of the nervous system. It is comprised of the central nervous system (the brain and spinal cord) and the peripheral nervous system
(Fig. 35.2). The peripheral nervous system is comprised of two parts: the voluntary or skeletal nervous system, which enables purposeful, conscious actions, like piano playing; and the involuntary or autonomic nervous system, which innervates organs to provide a major information conduit that regulates homeostasis. The autonomic nervous system normally functions without conscious involvement. There are two divisions of the autonomic nervous system: the sympathetic and parasympathetic. Most organs receive input from both of these autonomic divisions, which deliver information that adjusts organ output according to setpoints established in the brainstem and hypothalamus.
The neuron, the principle information-transmitting cell of the nervous system, communicates with other cells by action potentials that propagate unidirectionally along axons away from the neuronal cell body. Arrival of action potentials at the axon terminus causes the release of neurotransmitters into the synaptic cleft, the narrow space adjacent (< 20 nM) to the innervated neuron or somatic cell. This converts the electrical information carried in the axon into molecular (or pharmacologic) information, because secreted neurotransmitters bind to specific receptors expressed on the innervated cell. Ligand-receptor interaction leads to signal transduction that modulates the behavior of that cell in response to the arrival of a train of action potentials. Henry Dale, the Nobel Laureate discoverer of acetylcholine, recommended that autonomic nerves be described by the neurotransmitters they produce, not their function (ie, not as “sympathetic” or “parasympathetic”). This is an important point that will be emphasized again in this chapter. Autonomic neurons may be adrenergic (using catecholamines), cholinergic (using acetylcholine), or peptidergic (using neuropeptides) as their principle neurotransmitter.
The autonomic nervous system provides the principle communication pathway by which the nervous system rapidly adjusts specific cellular functions to maintain homeostasis. The nervous system can also communicate to other organ systems via the neuroendocrine organs, a humoral pathway that relies on the bloodstream to deliver the final signal. As compared to the neural communication pathway, the additional steps of hormone release and transit through the circulatory system means that neuroendocrinederived homeostatic regulation signals are delivered much more slowly than neural action potentials. The primary signal in both systems, however, is neural, because neuroendocrine signaling is initiated by neurons in the brainstem and hypothalamus that relay action potentials to the autonomic nuclei, and the pituitary gland, to regulate the release of pituitary hormones into the bloodstream. This modulates the endocrine organ output of glucocorticoids (GCs), thyroid hormones, catecholamines, and other circulating hormones that influence cellular function and metabolism in other organ systems. Neural signaling mechanisms adjust cellular responses in an extremely rapid time frame, whereas the neuroendocrine system establishes longer-term, more chronic homeostatic setpoints.
THE NERVOUS SYSTEM MAINTAINS ORGAN SYSTEM HOMEOSTASIS
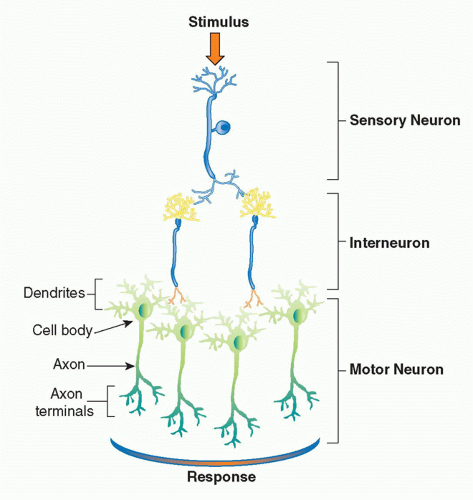
The basic physiologic unit that maintains organ homeostasis is the reflex. A neural reflex circuit is comprised of three components: a sensory arc, a relay or interneuron arc, and a motor arc
(Fig. 35.3). The sensory (afferent) neural path relays information to the central nervous system. Sensory neurons respond to pressure, vibration, temperature, and other molecular signals (eg, taste) that trigger action potentials that travel to the spinal cord or brainstem. Molecular products of infection, injury, and inflammation including bacterial endotoxin, interleukin (IL)-1, and tumor necrosis factor (TNF) can also activate specific receptors on sensory neurons, which initiate afferent action potentials that transfer information to the central nervous system. Within the central nervous system, interneurons receive the incoming signals from sensory nerves and transfer the information to other brainstem nuclei,
which coordinate the efferent, or outgoing, signals back to the organs. In a neural reflex, which operates extremely quickly, the signals are projected back to the organs via neuronal action potentials. In a neuroendocrine reflex circuit, the efferent neurons project to the hypothalamic-pituitary-adrenal axis, which in turn regulates circulating hormone levels over more prolonged periods of time.
A useful example of reflex circuits that maintain homeostasis of organ function is the acute neural regulation of heart rate and the chronic neuroendocrine regulation of blood pressure. The heart rate control circuit begins with pressuresensitive neurons that transmit information about heart rate to interneurons in the brainstem. These relay information to efferent brainstem nuclei that activate either adrenergic or cholinergic nerves that project back to the heart. The descending neural signals either increase or decrease heart rate in response to deviations from the heart rate setpoint as established in the brainstem. Blood pressure also activates sensory neurons, which initiate signals that modulate the renin-angiotensin system, and the hypothalamic pituitary axis. These neuroendocrine responses establish hormone levels that maintain chronic blood pressure levels. In both examples (heart rate and blood pressure), the reflex circuit is initiated by information traveling from the periphery to the nervous system; they differ in that neural signals elicit rapid changes in organ output, whereas the humoral pathway works to maintain long-term stability.
These reflex principles governing the cardiovascular and other relatively accessible organ system were established a long time ago. Only quite recently has it become possible to understand how these reflex principles operate in the more diffuse immune system. The remainder of this chapter reviews the neural and humoral mechanisms underlying the reflex control of immune homeostasis by the nervous system.
THE IMMUNE SYSTEM IS INNERVATED
As reviewed in
Chapter 3 in this volume, the immune system is comprised of circulating cells and resident or tissue fixed cells residing primarily in the spleen, liver, lymph nodes, thymus, lungs, intestines, central nervous system, and bone marrow. These anatomic sites are richly innervated by the autonomic nervous system via neurons that are adrenergic, cholinergic, and peptidergic.
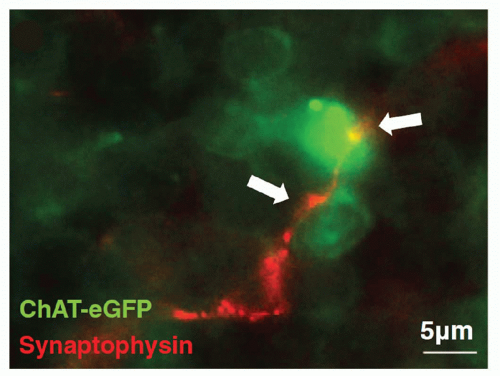
1,2,3 These neural circuits provide an anatomic and functional basis for direct neural communication between the nervous system and cells of the immune system. The majority of nerve fibers innervating the lymphoid tissues are adrenergic. They enter the lymphoid organs by traveling with the arterial vasculature; upon entering the tissue, they form networks that distribute to fields of lymphocytes and macrophages in the parenchyma. Innervation of lymphoid tissue is regional, primarily found in T-cell- and plasma-cell-rich zones, and lacking in nodular regions and zones of maturing B cells.
3Nerve terminals in the lymphoid tissues can undertake the appearance of a classic synapse on immune cells, which may be lymphocytes
(Fig. 35.4),
4 eosinophils, mast cells, and
macrophages.
3,5 These innervated cells express receptors for cholinergic (acetylcholine), adrenergic (norepinephrine and epinephrine), and peptidergic (vasoactive intestinal peptide, pituitary adenylate cyclase activating peptide, calcitonin gene-related peptide), and other (substance P, histamine and serotonin) neurotransmitters.
6 Release of neurotransmitters in response to action potentials propagated along these neurons specifically alters the metabolism, biochemistry, and gene expression patterns in the immune cells. Thus, neurotransmitter release converts the electrical signals propagated along neurons to the immune system into molecular or pharmacologic signals that exert discrete action on the responding immunocompetent cells.
Innervation of the immune cells within lymphoid organs is not a static phenomenon, because the number, density, and distribution of nerve fibers can undergo dynamic changes during immune responses. The molecular products of an immune response significantly influence the remodeling of nerve fibers and the expression of neurotransmitter receptors in lymphoid organs. Nerve fiber density is increased in the spleen and thymus of SCID mice, which lack functional T and B cells, and in nude mice, which lack T cells.
7,8,9 Reconstitution of SCID or nude mice with T-lymphocytes restores the normal pattern of innervations, indicating that immune cells modulate neural network formation. Additionally, systemic or regional inflammatory challenges lead to increase in nerve fiber distribution in the lymphoid organs such as thymus, lymph nodes, and spleen.
10,11,12 A reduction in splenic nerve fibers is observed during adjuvant arthritis in rodents, a finding that is accompanied by significant changes in spleen cytokine release.
13,14 Co-culture studies have established that neurons can selectively establish and maintain contacts with immune cells, and that cytokines (IL3, IL6, granulocyte macrophage-colony-stimulating factor-1, and nerve growth factor) derived from immune cells promote neurite expansion.
15,16,17 Integrins and other cell adhesion molecules also participate in the dynamic nature of nerve and immune cell interaction. Thus, the changing milieu in the immune system organs provides important signals that prune or expand the neural network to these organs. As will be explained in more detail subsequently, these changing neural networks in turn modify the ongoing immune response.
Together with other data that are beyond the scope of this chapter, these findings indicate that the nervous system is hardwired to the major organs of the immune system and is capable of conveying information that modulates immune cell function. These neural connections are plastic, operate rapidly, and are positioned to monitor and modify the immune response to products of infection, injury, or invasion.
THE NERVOUS SYSTEM MONITORS IMMUNE SYSTEM ACTIVITY
As the nervous system enjoys two routes to communicate with the immune system (neural and humoral), the immune system can also send information to the nervous system through both avenues (see
Fig. 35.1). Indeed, peripheral inflammation profoundly affects the function of the brain and central nervous system (CNS) though both neural and humoral mechanisms. Molecular products of inflammation alter neurogenesis, neural plasticity, learning, and memory. They also produce fever, anorexia, and acutephase responses. Cytokines and other immune system-derived molecules traveling in the circulation can influence neurons in the central nervous system through five mechanisms that circumvent the blood-brain barrier (BBB), the tight endothelial junctions that normally partition large or hydrophilic molecules in blood from the extracellular fluid compartment in the CNS. First, cytokines can pass through circumventricular regions of the brain, anatomic areas where the BBB is absent or incompetent.
18,19,20 Cytokines carried in the circulation to these brain regions can directly interact with neurons residing in the hind brain and brainstem. Second, cytokines can be actively transported across the BBB via a saturable transporter system.
21,22,23,24 This depends upon endothelial cell expression of specific cytokine binding transporter receptors that mediate the active chaperoning of the cytokines across the BBB and into CNS extracellular fluid.
25,26,27 Third, endothelial cells and perivasculature macrophages can bind circulating cytokines or pathogenassociated stimulatory molecules on the intraluminal side, and respond by secreting cytokines and other inflammatory mediators into the brain parenchyma on the adluminal side.
28,29,30 Fourth, activated immune cells, including monocytes/macrophages, can traverse the microvasculature, crossing from the circulation and entering the CNS parenchyma, where they can produce cytokines.
31 Fifth, cytokines can act on peripheral sensory nerve endings at the site of infection or injury, and the ascending action potentials can activate neurons and glial cells in the CNS to secrete cytokines.
32,33,34,35 Together, the net influence of these pathways is to provide mechanisms by which cytokines and other soluble immune system mediators can directly influence the activity of neurons in the CNS, which modifies the activity of neural signaling networks.
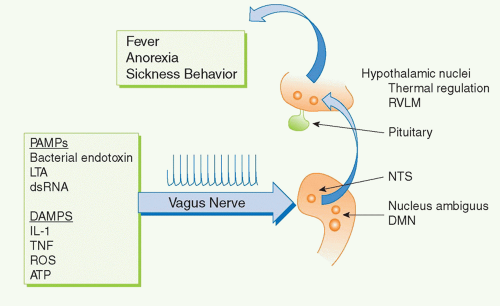
For example, several cytokines have pyrogenic activity, meaning that they activate fever responses. Early work on these mechanisms focused on the roles of blood-borne IL-1 and TNF entering the hypothalamus and altering the function of hypothalamic thermoregulatory nuclei that regulate body temperature. More recent discoveries revealed that the vagus nerve also communicates direct neural signals to mediate IL-1-induced fever. The vagus nerve connects the major body organs to the brainstem, and as many as 80-90% of the neurons that travel in the vagus nerve are afferent (sensory). This provides an important, rapid, and efficient neural route for communicating information about the organ milieu to the brain.
36 These afferent vagus nerve fibers express receptors for IL-1 and other inflammatory mediators.
32,37,38,39,40,41,42 Activation of these receptors by binding to IL-1 is an important mechanism for initiating fever, because cutting the vagus nerve prevents the development of fever in animals receiving of IL-1 by intra-abdominal injection.
38,43,44 Cytokine-activated afferent vagus nerve signals alter the function of thermoregulatory hypothalamic nuclei and the activity of other neurons in brainstem, hypothalamus, and limbic structures
(Fig. 35.5). Other sensory nerve fibers including,
for example, the glossopharyngeal nerves, can be activated by cytokines to mediate fever.
35,45,46 Neurophysiologic studies have demonstrated that administration of IL-1 in visceral organs stimulates the generation of action potentials that travel in the vagus nerve sensory arc, ascend to the brainstem, and return to the spleen and other organs.
47,48,49 Signals ascending to the nucleus tractus solatarius can be relayed throughout the brain, to the parabrachial nucleus, the thalamus, the paraventricular nucleus, the central nucleus of the amygdala, the insula cortex, the infralimbic cortex, the anterior cingulate cortex, and the medial prefrontal cortex. Thus, cytokines do not have to enter the bloodstream to mediate the onset of fever and other behavioral responses, because they can also activate these responses by binding to sensory neurons in distant peripheral tissues.
There are also immunocompetent cells residing within the CNS that can be activated to produce immunologically active molecules within the brain and spinal cord, where these mediators can exert their effects on neurons, locally. Numerous cell types in the CNS produce cytokines (and other inflammatory mediators) including astrocytes, microglia, lymphocytes, and dendritic cells. Immunocompetent cells are present in the choroid plexus, meninges, and brain parenchyma, and microglia form a regularly spaced network of resident cells throughout the CNS. Microglia are morphologically, phenotypically, and functionally related to the monocyte/macrophage cell lineage.
50,51,52 They are quiescent in healthy conditions, but respond rapidly to pathological conditions associated with infection, injury, invasion, or neuronal degeneration.
53 Microglial activation leads to cell proliferation, enhanced expression of cytokines and other inflammatory mediators, and recruitment to inflammatory sites.
50,54 Activation of micoglia and neurons by molecular products of inflammation induces the release of cytokines (e.g., IL1, IL6, and TNF) and other inflammatory mediators (e.g., prostaglandins and nitric oxide)
(Table 35.1). The local production of these mediators alters the activity of brainstem nuclei that mediate the onset of fever, anorexia, sickness behavior, acute-phase responses, and other neurologic responses.
Activated microglia upregulate the activity of phospholipase A2 and cyclooxygenases that catalyze the synthesis and release of prostaglandins and other eicosanoid mediators of inflammation. Other cells in the nervous system can be activated to release eicosanoid mediators during inflammation including astrocytes, neurons, and endothelial cells in the brain vasculature. Eicosanoid metabolites function in autocrine and paracrine signaling loops by binding to specific G-protein-coupled receptors expressed
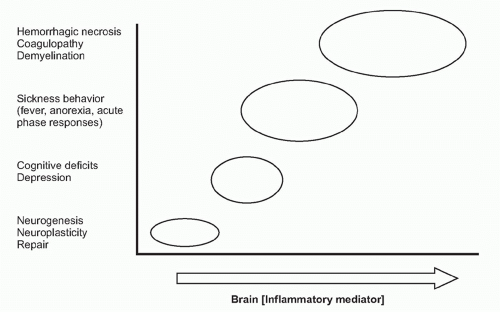
on neurons. Receptor-ligand signal transduction culminates on pathways that increase intracellular cyclic adenosine monophosphate (cAMP) concentrations. Eicosanoid signaling in neurons has been implicated in the development of fever, acute-phase protein responses, and impaired memory and learning. Infection, trauma, neurodegenerative diseases, and acute and chronic inflammatory diseases are all associated with increased release of cytokines and eicosanoids in the brain. Importantly, low-level eicosanoid production is necessary for neuronal homeostasis and is essential in the mechanisms underlying memory formation and neural plasticity
(Fig. 35.6). Eicosanoid levels that are either too high or too low impair memory development, thermoregulation, and feeding behavior. An inverted U-shaped doseresponse curve links IL-1 levels to memory function, which may be attributable to IL-1 signaling mechanisms that stimulate eicosanoid release in these mechanisms.
55,56 The neurophysiologic mechanisms underlying memory impairment in inflammatory conditions have been studied in brain slices, and addition of cyclooxygenase-2 inhibitors restores amyloid &bgr;-mediated impairment of long-term potentiation, the cellular neurophysiologic mechanism that links neuronal activity to memory formation.
57Activated microglia are also stimulated to produce increased levels of reactive oxygen species (ROS), including hydrogen peroxide, superoxide anion, and hydroxyl radicals. Like other inflammatory mediators, ROS are homeostatically regulated because their activities can be both deleterious and beneficial.
58,59 At low concentrations, free radicals play an important role in several cellular signaling systems. ROS regulate redox sensitive transcription factors such as NF-&kgr;B, AP-1, and nuclear factor of activated T-cells, and the redox state of tyrosine phosphorylated proteins, thereby having an impact on many transcriptional networks and signaling cascades important for neurogenesis. Low concentrations of ROS play a significantly beneficial role in neurogenesis. However, elevated ROS levels are extremely neurotoxic because they oxidize essential macromolecules such as enzymes and cytoskeletal proteins, lipids, and nucleic acids leading to neuronal death by apoptosis. ROS-mediated neuronal toxicity and degeneration is implicated in the pathogenesis of Alzheimer disease, Parkinson disease, and other neurodegenerative disorders.
60,61 Physiological ROS levels facilitate synaptic plasticity and cognitive function, but elevated ROS levels impair these functions. Thus, even within the CNS itself, the regulation of immune responses establishes a critical level of immunologic homeostasis that is crucial for normal, healthy nervous system function.