Although Suster and Moran20 have proposed a simpler classification schema that separates thymic tumors into three categories of thymic carcinoma: well-differentiated (WHO types A, AB, B1, and B2), moderately differentiated (WHO type B3), and poorly differentiated (WHO type C), the full WHO classification system remains more broadly accepted.
Currently, the terms noninvasive and invasive thymoma are preferred over benign and malignant designations. Noninvasive thymomas have an intact capsule, are mobile, and are easily resected, although they can be adherent to adjacent organs. In contrast, invasive thymomas invade surrounding structures and should be removed with en bloc resection of involved structures despite a benign cytologic appearance. Metastatic disease may occur in both noninvasive and invasive thymomas and is most commonly seen as pleural implants or pulmonary nodules. Metastases to extrathoracic sites, such as the liver, brain, bone, and kidney, rarely occur.21
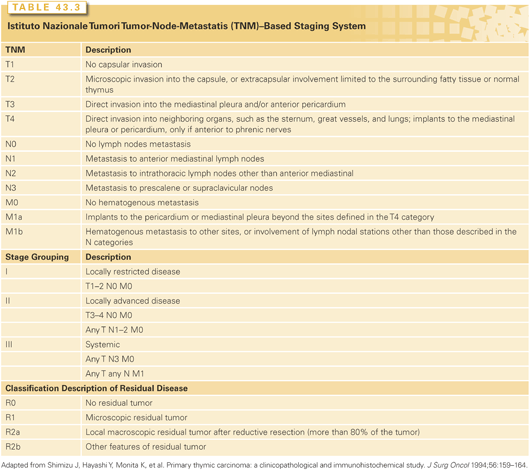
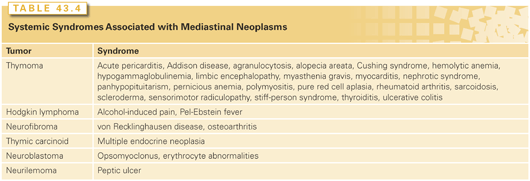
In 1981, Masaoka et al.22 developed a surgical staging system, shown in Table 43.2. An update of this series of 273 patients confirmed that both WHO histology and clinical staging were independently predictive of 20-year survival.23 The Groupe d’Etudes des Tumeurs Thymiques (GETT) has another surgically oriented staging system.24 The Istituto Nazionale Tumori system combines the Masaoka classifications in three distinct stage groupings (locally restricted, locally advanced, and systemic disease) and may better encompass all WHO subtypes.25 Although the Masaoka staging system26 and a tumor-node-metastasis (TNM) classification system27 (Table 43.3) also have been used in staging thymic carcinoma, their utility is largely unproven.
The histologic classification of thymic carcinoma was proposed by Levine and Rosai28 and revised by Suster and Rosai.4 Tumors are classified broadly as low or high grade. Low-grade tumors include squamous cell carcinoma, mucoepidermoid carcinoma, and basaloid carcinoma. High-grade neoplasms include lymphoepitheliomalike carcinoma and small-cell, undifferentiated, sarcomatoid, and clear-cell carcinomas.4,26,29,30 Although the histologic classification of thymic carcinomas was designed to be descriptive, correlations with prognosis have been made. For instance, low-grade tumors may have a more favorable clinical course (median survival rates of 25.4 months to more than 6.6 years) when compared with higher grade malignancies (median survival of only 11.3 months to 15.0 months).4,26
Molecular profiling of rare solid tumors, such as thymic neoplasms, has taken place over the past decade.31 Emerging analyses do suggest that mutations in genes of the epidermal growth factor receptor (EGFR) and KIT pathways have documented a progressive increase in observed genomic aberrations from WHO subtype A thymoma to WHO subtype C thymic carcinoma.32
Diagnosis
A meticulous history and physical examination, along with serologic and imaging studies, usually suggests the diagnosis. Although most anterior mediastinal masses are thymic malignancies, other etiologies also exist (Table 43.4). An improved pathologic analysis of image-guided percutaneous core needle biopsy specimens makes surgical biopsy rarely necessary.
Symptoms and Signs
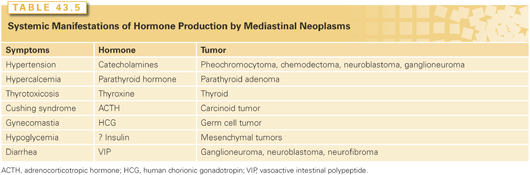
Approximately 40% of mediastinal masses are asymptomatic and discovered incidentally on routine chest imaging.1 The remaining 60% have symptoms related to either compression or direct invasion of adjacent mediastinal structures or to paraneoplastic syndromes. Asymptomatic patients are more likely to have benign lesions, whereas symptomatic patients more often harbor malignancies. Davis et al.33 found that 85% of patients with a malignancy were symptomatic, but only 46% of patients with benign neoplasms had identifiable complaints. The most common symptoms are chest pain, cough, and dyspnea. Superior vena cava syndrome, Horner syndrome, hoarseness, and neurologic deficits are less common and often signal a malignancy.33 Systemic syndromes associated with mediastinal neoplasms are shown in Tables 43.4 and 43.5.
Associated Systemic Syndromes
A myriad of associated systemic disorders may be identified in 71% of patients with thymomas, including autoimmune diseases (MG, systemic lupus erythematosus, polymyositis, myocarditis, Sjögren syndrome, ulcerative colitis, Hashimoto thyroiditis, rheumatoid arthritis, sarcoidosis, and scleroderma), endocrine disorders (hyperthyroidism, hyperparathyroidism, stiff-person syndrome, Addison disease, and panhypopituitarism), blood disorders (red cell aplasia, hypogammaglobulinemia, T-cell deficiency syndrome, erythrocytosis, pancytopenia, megakaryocytopenia, T-cell lymphocytosis, and pernicious anemia), neuromuscular syndromes (myotonic dystrophy, myositis, and Eaton-Lambert syndrome), as well as other disorders (hypertrophic osteoarthropathy, nephrotic syndrome, minimal change nephropathy, pemphigus, and chronic mucocutaneous candidiasis).34 Symptoms of one or more of these disorders may lead to the original discovery of the mediastinal tumor.
Myasthenia Gravis
MG is the most common autoimmune disorder associated with thymoma, occurring in 30% to 50% of patients.35 Younger women and older men usually are affected, with a female-to-male ratio of 2:1. Myasthenia is a disorder of neuromuscular transmission. The temporal association is variable and a prolonged interval between the diagnosis of MG and the development of a visible thymic tumor can occur.36 Symptoms (e.g., diplopia, ptosis, dysphagia, fatigue) begin insidiously and result from the production of antibodies to the postsynaptic nicotinic acetylcholine receptor at the myoneural junction. Ocular symptoms are the most frequent initial complaint, eventually progressing to generalized weakness in 80% of cases. The role of the thymus in MG remains unclear, but autosensitization of T lymphocytes to acetylcholine-receptor proteins or an unknown action of thymic hormones remains possible.37 The altered microenvironment may adversely impact the output of T-regulatory (Treg) cells, thus altering autoimmune homeostasis.38 Pathologic thymic changes are noted in 70% of MG patients, with lymphoid hyperplasia predominating and thymomas seen only in 15% of patients.37
The treatment of MG involves the use of anticholinesterase mimetic agents (i.e., pyridostigmine bromide [Mestinon]). In severe cases, plasmapheresis may be required to remove high antibody titers. Thymectomy has become an increasingly accepted procedure in the treatment of MG, although the indications, timing, and surgical approach remain controversial.39 Some improvement in MG symptoms frequently occurs after a thymectomy, but complete remission rates vary from 7% to 63%.39 Patients with thymomas do not respond as well to a thymectomy as MG patients without thymomas. Age 54 years and older and a symptom duration of less than 1 year are associated with poor outcomes40; however, MG does not necessarily portend a poor outcome.41
Red Cell Aplasia
Pure red cell aplasia, an autoimmune disorder, occurs in 5% of patients with thymomas.42 Of patients with red cell aplasia, 30% to 50% have associated thymomas. Affected patients are older than 40 years of age in 96% of cases. A bone marrow examination reveals an absence of erythroid precursors and, in 30% of cases, a poorly understood associated decrease in platelet and leukocyte numbers. A thymectomy has produced remission in up to 38% of patients. For patients with recurrent disease, octreotide and prednisone were effective in case reports.42,43
Hypogammaglobulinemia
Hypogammaglobulinemia is seen in 5% to 10% of patients with thymoma (Good syndrome), and 10% of patients with hypogammaglobulinemia have been shown to have thymoma. Recurrent sinusitis is a common associated symptom in such patients. Defects in both cellular and humoral immunity have been described, and many patients also have red cell hypoplasia.44 Thymectomy has not proven beneficial in this disorder.
Radiographic Imaging Studies
Imaging studies initially localize mediastinal neoplasms.45 The posteroanterior and lateral chest radiographs define the location, size, density, and calcification of a mass, which helps focus the initial diagnostic testing; however, an intravenous contrast-enhanced spiral computed tomography (CT) scan remains the best imaging modality to accurately assess the nature of the lesion (cystic versus solid), detect fat and calcium, determine the relationship to surrounding anatomic structures, and, in some instances, predict invasiveness of the tumors.46,47
Recent advances in electrocardiogram-gating and real-time magnetic resonance imaging (MRI) and angiography have dramatically increased the usefulness of this modality in the evaluation of mediastinal masses. Not only is it superior to CT in defining vascular involvement, but an MRI scan can also detect subtle differences in tumor contour, capsule clarity, and intratumoral signal (low), which correlate with the WHO classification of thymomas.48
Recently, the usefulness of positron emission tomography (PET) scans in the evaluation of thymic tumors has expanded significantly. In a study of 51 patients, Benveniste et al. suggested that 18-fluorodeoxyglucose (18F-FDG) uptake by PET/CT scans was higher in thymic carcinomas than thymomas. Additionally, higher focal 18F-FDG uptake correlated with B3 thymomas, and greater 18F-FDG avid tumor volume predicted higher stage tumors.49 In a contemporary study of 47 patients, Lococo et al. demonstrated that maximum standardized uptake values (SUVmax) and SUVmax/tumor size index, as determined by PET/CT, not only distinguished thymomas from thymic carcinomas, but also both parameters correlated with WHO malignancy grade and SUVmax predicted Masaoka stage.50 On serial PET/CT imaging, decreased 18F-FDG uptake in 56 patients with stage III/IV thymic epithelial malignancies treated after only 6 weeks of chemotherapy was shown by Thomas et al.51 to correlate with longer progression-free survival (11.5 versus 4.6 months; p = 0.044) and a trend toward longer overall survival (31.8 versus 18.4 months; p = 0.14).
Serology and Chemistry
Many germ-cell neoplasms release chemical markers into the serum that may be measured to confirm a diagnosis, evaluate the response to therapy, and monitor for tumor recurrence. Lactate dehydrogenase, α-fetoprotein (AFP), and human chorionic gonadotropin-β (β-hCG) are common tumor markers that should be obtained in male patients with anterior mediastinal masses. Also, adrenocorticotropic hormone, thyroid hormone, and parathormone may help differentiate certain mediastinal masses (see Table 43.4).
Invasive Diagnostic Tests
An accurate histologic diagnosis is essential for appropriate treatment of nearly all mediastinal neoplasms. Although some patients may still require open surgical biopsies, CT- or ultrasound-guided percutaneous needle biopsy is now standard in the initial evaluation of mediastinal masses.10 Although fine-needle specimens may distinguish carcinomas from benign pathology, core biopsies are necessary for most mediastinal neoplasms, especially lymphoma and thymoma. Recent series report diagnostic yields for percutaneous needle biopsy in excess of 90%.52 Complications include simple pneumothorax (25%), hemoptysis (7% to 15%), and pneumothorax, requiring chest tube placement (5%).52 In some circumstances, fine-needle aspiration of posterior and middle mediastinal tumors can be performed endoscopically using transesophageal ultrasonography.53
Surgical procedures occasionally are still required in the diagnosis of mediastinal tumors. A mediastinoscopy is a relatively simple procedure with a diagnostic accuracy of more than 90% for biopsies of the upper middle and, in some surgeons’ hands, the anterior and posterior mediastinum.54 Anterior parasternal mediastinotomy (Chamberlain procedure) yields a diagnosis in 95% of anterior mediastinal masses and may be accomplished under local anesthesia.54,55 A thoracoscopy is a minimally invasive procedure that provides a diagnostic accuracy of nearly 100% in most areas of the mediastinum.54 Currently, thoracotomy rarely is necessary solely as a diagnostic procedure.
Management by Stage
Thymomas, albeit slow growing, should be considered potentially malignant neoplasms. Surgery, radiation, and chemotherapy all may play a role in their management.41,56 Few prospective, well-designed clinical trials in the management of thymomas have been conducted, particularly evaluating the role of surgery and radiotherapy; however, the newly formed International Thymic Malignancy Interest Group is planning cohort studies to help guide diagnostic and therapeutic interventions.57
Masaoka Stage I/II Thymoma
Complete surgical resection is the mainstay of therapy for stage I/II thymomas and is the most important predictor of long-term survival.58–61 Although a median sternotomy with a vertical or submammary skin incision is most commonly used, bilateral anterolateral thoracotomies with transverse sternotomy, or the clam-shell procedure, is useful with advanced or laterally displaced large tumors. Recently, the use of minimally invasive surgery in stage I and II thymomas has expanded dramatically. One study comparing 76 thorascopic thymectomies to 44 transsternal resections reported a shorter hospital stay with the thoracoscopies.62 Similarly, two reports of robotic-assisted surgery, including a multicenter European study, were associated with less blood loss, fewer complications, and a shorter hospital stay, as well as similar operative times and short-term outcomes compared to sternotomies.63,64 Although randomized, prospective clinical trials are still lacking, it is highly likely that minimally invasive surgery in the hands of experienced surgeons can produce identical oncologic outcomes with less morbidity than open surgery, as has been shown in other thoracic tumors, such as lung and esophageal cancers.
During any surgery, a careful assessment of areas of possible invasion and adherence should be made. Extended total thymectomy, including all tissue anterior to the pericardium from the diaphragm to the neck and laterally from one phrenic nerve to the other, including en bloc pericardium, phrenic nerve, chest wall, lung, and diaphragmatic resection (with reconstruction) in up to two-thirds of cases in order to achieve an R0 resection is recommended in all good performance status patients.41,58–60 Operative mortality is less than 3% in experienced centers.41
In the past, ionizing radiation has been used to treat various stages of thymomas. Furthermore, modern imaging, three-dimensional treatment planning, and delivery techniques have allowed thoracic radiotherapy to be prescribed in a safer fashion than noted in the past century. Radiation therapy is delivered in doses ranging from 30 to 60 Gy in 1.8- to 2.0-Gy fractions for 3 to 6 weeks.58,65,66 There are suggestions of a dose–response relationship with local control in some patients, albeit from retrospective data, although it is not clear that doses exceeding 60 Gy offer any consistent advantage67,68; however, completely resected and microscopic residual disease can be well controlled with only 40 to 45 Gy.61,69 Emerging data suggest that certain histologic subtypes (WHO type B1 and B2) are more likely to respond to radiotherapy compared to others subtypes (WHO type B3), suggesting that the response is limited to the lymphocytic and not the epithelial cell component of the tumors.41,70–72 Gating techniques to minimize respiratory variation and intensity-modulated radiation therapy are new techniques that can minimize the dose heterogeneity, increase total dose and fraction size, and minimize toxicity.69,73,75
Masaoka Stage III/IV Thymoma
The role of subtotal surgical resection, or debulking surgery, in stage III and IV disease remains highly controversial.76 Several studies have documented improved 5-year survival rates after subtotal resection compared to a biopsy alone.58,61 Another study suggested no survival advantage to debulking surgery followed by radiation when compared with radiation alone, and a more recent report reached the opposite conclusion.76 The use of surgery in recurrent disease remains to be defined.
Radiation therapy may be beneficial in selected patients with locally advanced disease.61,65,67,77 Large variations in the amount of tumor treated, radiation delivered, and tumor biology, however, make interpretation of these results difficult.61,67,78,79
Cytotoxic chemotherapy has been used with increasing frequency in the treatment of invasive thymomas.80 Both single-agent and combination therapy have demonstrated activity in the adjuvant and neoadjuvant settings. Doxorubicin, cisplatin, ifosfamide, corticosteroids, and cyclophosphamide all have been used as single-agent therapies.81 The most active agents are cisplatin, doxorubicin, ifosfamide, and corticosteroids; however, only a few single agents, such as cisplatin, ifosfamide, pemetrexed, gefitinib, and imatinib, have undergone formal phase II trials.
A number of molecular targets have been identified in thymic tissue.83 Overexpression of the EGFR has been found in more than two thirds of patients, mostly WHO B2 and 3 subtypes.84–86 The overexpression of c-kit is common in thymic carcinoma, although c-kit mutations are less frequent.85,87,88
Thymic Carcinoma
The optimal treatment of thymic carcinoma remains undefined, but currently, a multimodality approach, including surgical resection, postoperative radiation, and chemotherapy, is recommended.89 Initial surgical resection followed by radiation has been used in most studies.4,26,27,29,90 Complete resection should be attempted, but often is not possible.26,91 One analysis noted a 9.5-month median survival after resection and postoperative photon-beam radiation therapy,90 with a trend toward improved survival in other studies.4,26,29 Chemotherapy with cisplatin-based regimens similar to those used with thymomas have produced variable responses in a small number of patients.4,26,29 Combinations of doxorubicin, cyclophosphamide, and cisplatin also have generated partial responses, as has the combination of 5-fluorouracil and leucovorin in recurrent disease. Use of neoadjuvant chemotherapy has been reported in a small number of patients.89,92
Results of Treatment
Thymoma
In nearly 700 patients in the SEER database treated between 1973 and 1998, advanced disease was associated with decreasing survival.42 According to various retrospective series, the 5- and 10-year survival rates for stage I, III, and IV tumors are reported to be 89% to 95% and 78% to 90%; 70% to 80% and 21% to 80%; and 50% to 60% and 30% to 40%, respectively.58,93 Ten-year disease-free survival rates of 74%, 71%, 50%, and 29% also have been reported for stage I, II, III, and IV disease, respectively.58 Long-term results from an experienced Indiana University group has yielded a 66% 1-year overall survival.41 Although Maggi et al.58 reported a 10% overall recurrence rate in 241 patients, less than 5% of noninvasive thymomas and 20% of invasive thymomas were noted to recur. A large Japanese multi-institutional experience with 1,320 patients reported 5-year survival rates of 100%, 98.4%, 88.7%, 70.6%, and 52.8% for Masaoka stages I, II, III, IVa, and IVb, respectively.59 Although MG once was considered an adverse prognostic factor, this is no longer the case because of improved perioperative care, and in fact, MG actually may be associated with an improved survival owing to earlier tumor detection.41,94 Complete surgical resection of thymomas is associated with an 82% overall 7-year survival rate, whereas survival with incomplete resection is 71%, and with biopsy is only 26%.58 Survival after a complete tumor resection has been similar in patients with noninvasive and invasive thymomas in several studies.59,60 Patients with MG and thymoma have a 56% to 78% 10-year survival rate and a 3% recurrence rate with 4.8% (1.7% since 1980) operative mortality after an extended thymectomy.61,95 Rarely, a syndrome of myasthenia crisis may occur following surgery and may lead to increased perioperative morbidity.95
Although the data on whether outcomes are related to WHO subtype is inconclusive,41,71,72 it may be that when coupled with other factors such as completeness of resection, the WHO subtype may be prognostic in some patients.96
For example, in stage I thymomas, adjuvant radiotherapy has been administered but has not improved on the excellent results with surgery alone (more than 80% 10-year survival rate).58,61 In stage II and III invasive disease, adjuvant radiation can decrease recurrence rates after complete surgical resection from 28% to 5%.67 In addition, Pollack et al.65 reported an increase in 5-year disease-free survival for stage II to IVa from 18% to 62% with the addition of adjuvant radiation, despite a 50% infield relapse rate in patients recurring with prior radiation. Stage II patients with cortical tumors97,98 and invasion of pleura or the pericardium are most likely to benefit from postoperative radiation.77 Preoperative radiotherapy for extensive tumors has been reported in limited studies that suggest a decreased tumor burden and potential for tumor seeding at the time of surgery, although multivariate analyses suggested that chemotherapy may have played a role.58,61 Data suggest that not all Masaoka stage II patients may necessarily require postoperative radiotherapy (PORT).56,101 Indeed, a review of SEER registry data from 1975 to 2003 demonstrated a worse cancer-specific survival for patients with resected, localized thymoma who received PORT compared to those without (91% versus 98%; p = 0.03). For patients with regional disease, PORT had a slight but nonstatistically significant difference (91% versus 86%; p = 0.12).102 The absence of statistically significant therapeutic efficacy for PORT, despite the suggestion of a lower local recurrence rate (i.e., 0% versus 8%) again has been confirmed in two recent studies.102
Masaoka Stage III/IV Thymoma
The role of subtotal surgical resection or debulking surgery in stage III and IV disease remains highly controversial.76 Several studies have documented 5-year survival rates from 60% to 75% after subtotal resection and 24% to 40% after biopsy alone.58,61 Although one study did suggest no survival advantage to debulking followed by radiation when compared with radiation alone, another more recent experience reached the opposite conclusion.76 The use of surgery in recurrent disease remains to be defined. Maggi et al.58 reported a 71% 5-year survival rate in 12 surgery patients and a 41% survival rate in 11 patients treated with radiation and chemotherapy alone. Prolonged tumor-free survival also was reported by Kirschner74 in 23 patients. However, Urgesi et al.78 noted a 74% 5-year survival rate in 11 patients undergoing surgery and radiation, compared with 65% in 10 patients treated with radiation alone (not statistically different). In 71 patients with thymoma (WHO types A and B3) with stage I to IVA disease, 53 (75.3%) patients are alive and free of disease, with 6 additional patients alive with disease with a mean follow-up of 66 months.41
Radiation therapy may be beneficial in selected patients with locally advanced disease.61,63,65,68,77 Radiotherapy after incomplete surgical resection produces local control rates of 35% to 74% and 5-year survival rates ranging from 50% to 70% for stage III and 20% to 50% for stage IVa tumors.61,66,67 In addition, Ciernik et al.67 and others78 have reported similar survival rates (87% 5-year and 70% 7-year) in patients treated with radiation alone compared with partial surgical resection and adjuvant radiation in small numbers of stage III and IV patients and patients with intrathoracic recurrences. Weksler et al.105 reviewed SEER data on 322 patients with stage III thymoma who received postoperative adjuvant radiation and found that by multivariate analysis, disease-specific survival was improved (p = 0.049) but overall survival was not. However, large variations in the amount of tumor treated, radiation delivered, and tumor biology in particular make interpretation of these results difficult at best.61,67,78,79
Cytotoxic chemotherapy has been used with increasing frequency in the treatment of invasive thymomas.80 Both single-agent and combination therapy have demonstrated activity in the adjuvant and neoadjuvant settings. The most commonly used active agents are cisplatin and doxorubicin; however, only a few single agents, such as cisplatin, ifosfamide, pemetrexed, gefitinib, and imatinib, have undergone formal phase II trials.80 Cisplatin at doses of 100 mg/m2 has produced complete responses lasting up to 30 months, but lower doses (50 mg/m2) have associated response rates of only 10%.81 In 13 earlier patients, ifosfamide (with mesna) was given at a single dose of 7.5 g/m2 or as a continuous infusion of 1.5 g/m2 per day for 5 days every 3 weeks and resulted in five (38.7%) complete and one (7.7%) partial responses.106 Varying regimens of corticosteroids have shown effectiveness in the treatment of all histologic subtypes of thymoma (with and without myasthenia), with a 77% overall response rate in limited numbers of patients.82
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree