Natural Killer Cells
Learning Objectives
• Identify the phenotypic characteristics of natural killer (NK) cells
• Recognize the mechanism by which NK cells recognize aberrant or infected cells
• Differentiate between the two major classes of activation and inhibition molecules of NK cells
• Identify the major ligand CD94–NKG2 complex
• Compare and contrast the physiologic roles of the subpopulations of NK cells
• Contrast the roles of NK cells in viral infections and tumor surveillance
• Explain the mechanism involved in antibody-dependent cellular cytotoxicity (ADCC)
• Compare and contrast the physiologic roles of NK cells and NKT cells
• Recognize the phenotypic characteristics of NKT cells
• Recognize the two major subsets of NKT cells
• Discuss the role of alpha galactosyl ceramide (α-GalCer) in NKT cell stimulation
• Compare the roles of IL-12 and IL-13 in tumor immunity
• Explain the relationship between type I NKT cells and asthma
• Identify the roles of type II NKT cells in viral and autoimmune diseases
• Identify the nature of the genetic defect in Chediak-Higashi syndrome (CHS)
Introduction
Natural killer (NK) cells are defined by the expression of CD16 and CD56 surface molecules. In peripheral blood, two populations of NK cells are present. One population comprises large granular lymphocytes that are phenotyped as CD3–, CD16+, CD56+, and CD94+ cells. Since they do not express T or B cell markers, they are considered to be of a third lymphocyte lineage. The second population of NK cells comprises T cells that express both T cell and NK cell markers (CD3+, CD16+, CD56+ or CD3+, CD16–, CD56+). These cells are known as natural killer T cells (NKT cells) to differentiate them from large granular lymphocytes.
NK and NKT cells have different functions. NK cells are part of the innate cell-mediated response to infected or tumor cells. They recognize and lyse cells with downregulated human leukocyte antigen (HLA) class I molecules. In contrast to classic NK cells, NKT cells have limited cytolytic capability and require antigen presentation. Emerging evidence also suggests that NKT cells are involved in immediate allergic reactions, suppression of auto-reactive lymphocytes, and tumor immunity. Activation or inhibition of NK and NKT cells is dictated by interactions between cell receptors and HLA class IB molecules or CD1 molecules on target cells.
Natural Killer Cell Receptors
CD94–NKG2 Receptors
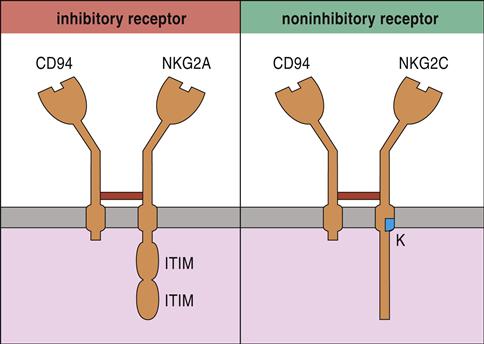
The most studied NK cell receptor is CD94—a type 2 “lectin-like” receptor expressed on both NK cells and cytotoxic T cells (CTLs). To create an active receptor, CD94 pairs with NKG2A, B, C, E, or F. NK cell function is dictated by different pairings between CD94 and NKG family members. For example, NKG2A and NKG2B dimerize with CD94 and inhibit NK cell function. Conversely, CD94–NKG2C dimers activate NK cells (Figure 21-1).
Killer Cell Immunoglobulin-Like Receptors
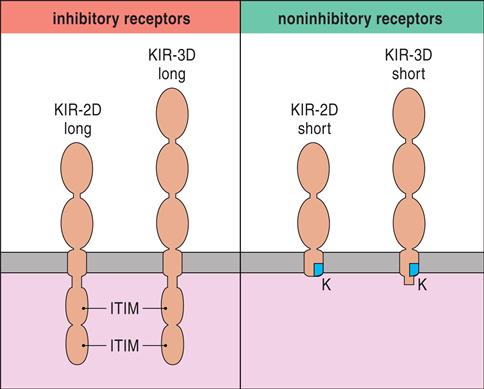
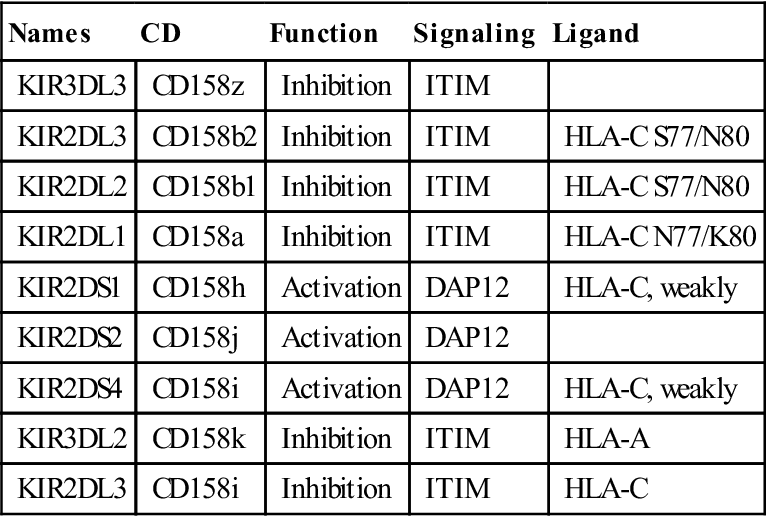
Killer cell immunoglobulin-like receptors (KIRs) are encoded by a family of 15 polymorphic genes and two pseudogenes on chromosome 19. KIRs are membrane anchor proteins that possess either two (KIR2DS) or three (KIR3DS) immunoglobulin domains (Figure 21-2).
Within each subfamily, KIRs that inhibit or activate NK cells are present (Table 21-1).
Table 21-1
Immune Receptors Encoded by Genes in the Leukocyte Receptor Complex Region
| Names | CD | Function | Signaling | Ligand |
| KIR3DL3 | CD158z | Inhibition | ITIM | |
| KIR2DL3 | CD158b2 | Inhibition | ITIM | HLA-C S77/N80 |
| KIR2DL2 | CD158b1 | Inhibition | ITIM | HLA-C S77/N80 |
| KIR2DL1 | CD158a | Inhibition | ITIM | HLA-C N77/K80 |
| KIR2DS1 | CD158h | Activation | DAP12 | HLA-C, weakly |
| KIR2DS2 | CD158j | Activation | DAP12 | |
| KIR2DS4 | CD158i | Activation | DAP12 | HLA-C, weakly |
| KIR3DL2 | CD158k | Inhibition | ITIM | HLA-A |
| KIR2DL3 | CD158i | Inhibition | ITIM | HLA-C |
Modified from Cooper MD, Lewis LL, Conley, ME, et al: Immunodeficiency disorders. American Society of Hematology Education Program, pp. 314–330, 2003. Hematology 2003, The American Society of Hematology.
Target Cell Recognition Molecules
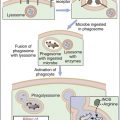
NK cells do not detect aberrant or infected cells. Rather, they recognize cells that lack HLA class I molecules. This observation has led to the development of the “missing self” hypothesis of NK cell activation. The hypothesis suggests that NK cells can kill normal cells but are prevented from doing so by the presence of inhibitory factors such as HLA class I markers. Downregulation of class I molecules allows NK cells to engage other molecules that either activate or inhibit the NK cell.
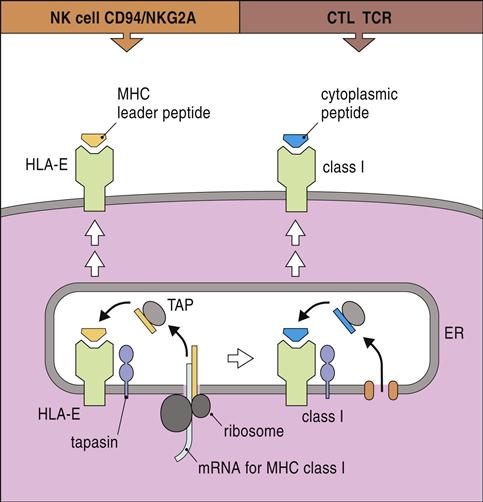
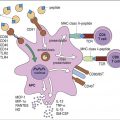
HLA-E, a nonclassic HLA locus, is the natural ligand for CD94–NKG2A and B complexes. HLA-E binds the peptide leader sequence from HLA-G. The HLA-G sequence is encoded upstream of the open reading frame for the α-chain. In the cytoplasm, the N-terminal fragment of the leader sequence is released after cleavage by signal peptidases and endoplasmic reticulum (ER) proteases. The small leader peptide is loaded into the binding groove in the ER and transported to the cell surface (Figure 21-3).