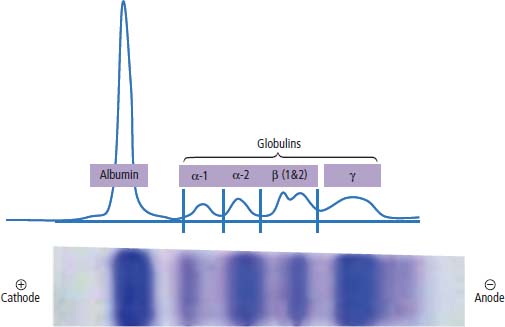
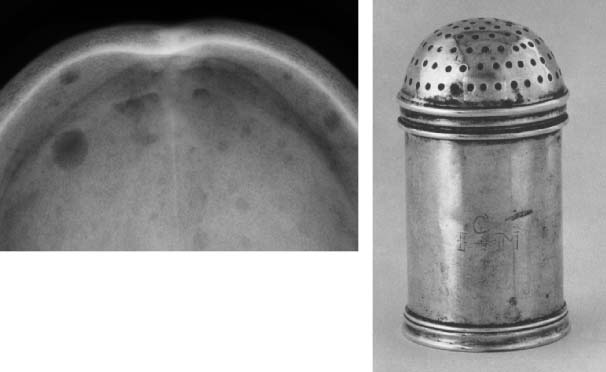
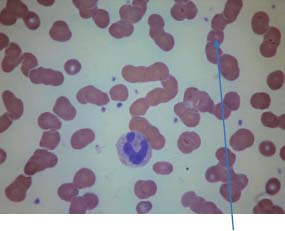
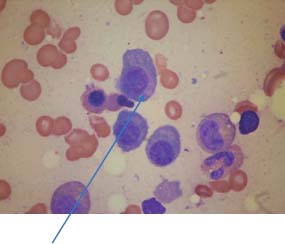
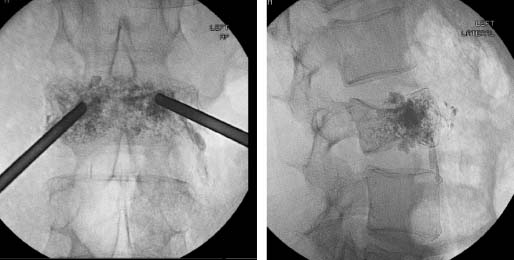
31 Myeloma is a relatively common haematological malignancy; 4792 people were diagnosed and 2693 died of myeloma in the United Kingdom in 2011. There is an equal sex distribution and an increasing incidence with age (Table 31.1). Risk factors for myeloma are remarkably absent. There are minor increases in risk with family history, pernicious anaemia, HIV infection and organ transplantation, although none of these associations are very strong. Multiple myeloma is a B-cell neoplasm characterized by the proliferation of plasma cells that synthesize and secrete monoclonal immunoglobulins or fragments thereof. It is believed to evolve from an asymptomatic pre-malignant clonal condition of plasma cells known as monoclonal gammopathy of undetermined significance (MGUS). MGUS affects 3% of the population over 50 years of age and progresses to myeloma at a rate of 1% per year. MGUS is commonly associated with chromosomal translocations of the immunoglobulin heavy chain genes often with cyclin D genes. Those MGUS that lack IgH translocations often have chromosomal trisomy. The subsequent transformation from MGUS to myeloma is associated with further genetic alterations that are less uniform. Amongst these, second hit random alterations are mutations of Ras and p53genes and methylation of p16 and cyclin D kinase inhibitor genes. T-lymphocytes secrete the cytokine interleukin 6 (IL-6), which appears to be an essential growth factor for myeloma cells in culture. Excessive secretion of IL-6 occurs in myeloma from stromal cells and this may be a primary cause for the condition. Fashions in molecular biology change their focus with time and the current vogue includes micro RNAs (miRNAs) as a means of regulating gene function. miRs are small 22 nucleotide non-coding RNAs that regulate gene function by base pair binding complementary messenger RNA (mRNA) and hence preventing its translation by ribosomes into proteins. The human genome is thought to encode over 1000 miRs. In myeloma, miR-21 and miR-106b are thought to be overexpressed and by regulating the tumour suppressor p53, interleukin-6 (IL6) and its downstream JAK-STAT signalling lead to deregulation of complex pathways. Similarly, angiogenesis in myeloma is postulated as being affected by changes in miR-16 which targets vascular endothelium growth factor (VEGF). The destructive bone lesions that are seen in myeloma are thought to be due to dysregulation of the osteoprotegerin Rankl (receptor activator of nuclear factor kappa B ligand) system. Rankl is the ligand for osteoprotegerin and is released in myeloma by the malignant plasma cells and bone marrow stroma, leading to osteoclast activation and hence osteolysis. The osteolytic bone lesions of myeloma are best seen on plain X-rays rather than bone scans as they generally lack osteoblastic activity. They appear as punched-out lesions, including the classical “pepper pot” appearance of the skull X-ray (Figure 31.2), and the bone breakdown releases calcium and may cause hypercalcaemia. Table 31.1 UK registrations for myeloma cancer 2010 In addition to the local effects of myeloma on bone, patients with myeloma may present with hypercalcaemia, renal failure, bone marrow failure or rarely the effects of excess immunoglobulin levels in the blood. Patients with myeloma most often present with significant bone pain due to the lytic lesions that characterize this disease. Vertebral collapse is often a feature of presentation and this may lead in a dramatic fashion to cord compression. Patients with myeloma may present with symptoms of hypercalcaemia, which every medical student reading this chapter should be able to describe. Renal failure is a common feature of myeloma and may arise through a number of mechanisms. Hypercalcaemia can be one of the precipitating factors for renal failure. The other causes include amyloidosis (AL) (amyloid containing immunoglobulin light chains), precipitation of Bence–Jones protein (urinary free light chain paraprotein) and direct infiltration and infection. An excess of immunoglobulin may cause the hyperviscosity syndrome, which is more common with an IgG myeloma than an IgM myeloma. This is explained by the fact that a far greater proportion of patients have IgG than IgM myelomas, which represents just 0.5% of all myeloma cases. The symptoms of hyperviscosity include spontaneous bleeding, retinopathy and neurological symptoms ranging from headache to coma. Hyperviscosity can also occur with high blood cell counts in leukaemia and polycythemia. The raised paraprotein levels may cause other problems, including peripheral neuropathy. Marrow infiltration with an excess of plasma cells leads to a decrease in numbers of other marrow constituents, causing anaemia, thrombocytopenia and neutropenia. This in turn has consequences for both the presentation and the clinical features of the disease as it evolves. The investigation of myeloma is relatively simple. It requires the examination of the peripheral blood, paraprotein levels (serum electrophoresis and immunofixation and serum-free light chain assays) (Figure 31.1), blood count, β2-microglobulin levels, renal function and calcium levels (see Figures 31.3 and 31.4), assessment of the bone marrow, examination of the urine for Bence–Jones protein urea and a skeletal survey (Table 31.2). Bone scanning is of low diagnostic value in myeloma. Myeloma is staged and the staging has prognostic value. Two systems are used, that of Durie and Salmon and the simpler International Staging System (ISS) (Tables 31.3 and 31.4). Before treatment is contemplated the diagnosis of multiple myeloma must be established and differentiated from MGUS and smouldering myeloma (Table 31.2) which do not require therapy. The initial treatment of myeloma requires stabilization of the patient and correction of renal function abnormalities and hypercalcaemia (Table 37.4). The patient is started on allopurinol and may require hydration or transfusion. Hypercalcaemia is treated with bisphosphonates, steroids and by rehydration. Where there is significant bone pain, which is poorly responsive to opiates, radiotherapy may be required. A single fraction treatment will alleviate bone pain in approximately 80% of patients. Anaemia may require transfusion and significant hyperviscosity needs treatment by plasmaphoresis. Plasmaphoresis involves taking blood, removing abnormal proteins from the plasma by extracorporeal filtration and then returning the blood to the patient on a continuous circuit. Plasmaphoresis provides transient reductions in paraprotein levels, allowing relief of acute medical complications of the paraproteinaemia as drug treatment takes effect. Figure 31.1 Normal serum protein electrophoresis. Figure 31.2 The pepper pot skull medical metaphors: (cf. Figures 5.5, 11.1 and 28.13). Skull radiograph of a 52-year-old man with multiple myeloma showing multiple, well-defined lucencies that are fairly uniform in size, unlike bone metastases which usually vary in size. Figure 31.3 Peripheral blood film showing rouleaux formation with erythrocytes stacked up on each other and a single neutrophil. Rouleaux are found at high levels in the blood of proteins such as fibrinogen or γ-globulin. They are particularly prominent in diseases that cause a very high erythrocyte sedimentation rate (ESR), such as multiple myeloma, cancers, chronic infections (e.g. TB) and connective tissue diseases. Figure 31.4 Bone marrow aspirate of myeloma showing plasma cells with large eccentric nuclei and basophilic cytoplasm. Table 31.2 Diagnostic criteria for plasma cell proliferations Chemotherapy for myeloma has a 50-year history, beginning with the use of alkylating agents such as melphalan and cyclosphosphamide. Nowadays, the initial treatment of multiple myeloma depends on a risk stratification into high-risk, intermediate-risk and low-risk disease based on cytogenetics or gene expression profiling signatures. High-dose chemotherapy with hematopoietic stem cell transplantation has become the preferred treatment for patients under the age of 65. Prior to stem cell transplantation, these patients receive an initial course of induction chemotherapy. The most common induction regimens used are thalidomide–dexamethasone, bortezomib-based regimens and lenalidomide–dexamethasone. Unfortunately, many patients are older and frailer and are not eligible for transplantation; here the standard of care has been chemotherapy with melphalan and prednisone, bortezomib containing regimes or lenalidomide and dexamethasone. Bortezomib inhibits proteosomes, the cellular organelles that identify tagged proteins in the cytoplasm and break them down. It is not quite clear how thalidomide and lenalidomide work but they seem to have both immunomodulatory and anti-angiogenic actions. Both are potent teratogens and it is essential that they are not prescribed to women who could become pregnant. Table 31.3 Durie and Salmon staging system for myeloma Table 31.4 International staging system All myeloma patients are at risk of pathological bone fractures and should modify their lifestyles to avoid this risk. Patients with skeletal lesions or osteopenia should be given bisphosphonates which reduce the risk of skeletal bone events (Figure 31.5). Figure 31.5 Vertebroplasty. Myeloma patients with painful compression fractures of vertebral bodies may have bone cement injected percutaneously. This procedure is only safe when there is no epidural disease or retropulsion of bone fragments into the spinal cord. There has been recent progress in the development of new treatments for myeloma that have gone beyond thalidomide analogues and proteasome inhibitors. These developments are in the area of the “Ab’s” rather than the “Ib’s”. Daratumumab, a humanized antibody to CD38, has been investigated in both phase 1 and phase 2 drug development trials. This drug was granted breakthrough drug status by the FDA in 2014 because of the benefits seen in myeloma patients in reducing M band levels and bone marrow infiltration with plasma cells. This rapid approval process in the United States will no doubt contrast with UK approval processes, or should we more accurately write, “disapproval processes”? We fear, from past experience, that NICE will turn down this drug for approval on the basis of some spurious cost–benefit analysis, after prolonged delays, whilst myeloma patients die waiting. Siltuximab is a chimeric monoclonal antibody to IL6, which is an effective new treatment for Castleman’s disease that offers hope in myeloma where it reduces M band levels and is undergoing assessment in this condition currently. It is to be hoped that these new treatments for myeloma will continue to build on to the near doubling of survival in myeloma that has already come with advances in drug treatment. It is the authors’ ambition to see the blunderbuss of high-dose chemotherapy and bone marrow transplantation replaced by treatment targeting the molecular biology of this disease. Case Study: The pensioner who found washing up painful.
Myeloma
Epidemiology
Pathogenesis
Percentage of all cancer registrations
Rank of registration
Lifetime risk of cancer
Change in ASR (2000–2010)
5-year overall survival
Female
Male
Female
Male
Female
Male
Female
Male
Female
Male
Myeloma
1
2
17th
15th
1 in 155
1 in 120
+8%
+13%
37%
37%
Presentation
Investigations
Treatment
Multiple myeloma (all three criteria must be met)
Presence of a serum or urinary monoclonal protein
Presence of clonal plasma cells in the bone marrow or a plasmacytoma
Presence of end organ damage felt to be related to the plasma cell dyscrasia
Smoldering multiple myeloma (SMM) (both criteria must be met)
Serum monoclonal protein >3 g/dL and/or >10% to <60% bone marrow clonal plasma cells
No end organ damage related to the plasma cell dyscrasia
Monoclonal gammopathy of undetermined significance (MGUS) (all three criteria must be met)
Serum monoclonal protein <3 g/dL
Bone marrow plasma cells <10%
No end organ damage related to plasma cell dyscrasia
Cell mass category: requirements
High (stage III): one of A, B, C or D
Low (stage I): all of A, B, C and D
Intermediate (stage II)
Haemoglobin (pre-transfusion)
A
<85 g/L
>100 g/L
Neither I or III
Serum calcium
B
>3 μmol/L
Normal
M component
C
IgG >7 g/dL or IgA >5 g/dL
IgG <5 g/dL or IgA <3 g/dL
Urinary monoclonal protein (Bence–Jones protein)
D
Urine monoclonal protein excretion >12 g/day
Urine monoclonal protein excretion <4 g/day
Bone lesion on skeletal survey
E
Advanced lytic disease
None/solitary lesion
Stage
Criteria
Stage I
Beta-2 microglobulin <3.5 mg/L and serum albumin >35 g/L
Stage II
Neither stage I nor stage III
Stage III
Beta-2 microglobulin >5.5 mg/L
 ONLINE RESOURCE
ONLINE RESOURCE
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


