Introduction
The myelodysplastic syndromes (MDS) are a heterogeneous group of clonal stem cell disorders characterized by dysplasia, ineffective haematopoiesis and potential risk of transformation into acute leukaemia.1, 2 These disorders are sporadic and arise de novo or may result following toxin, radiation and chemotherapy exposure (secondary MDS).3, 4 MDS primarily affects older patients, with an increased incidence with advancing age.5, 6 Disease onset prior to the sixth decade is uncommon, although not unheard of, especially with treatment-related or secondary MDS. As the demographics in developed countries shift towards older patient populations due to increased longevity and better quality of healthcare and more people are receiving intensive chemotherapy, the prevalence of MDS will likely increase.
MDS may easily be overlooked in elderly patients. It can present simply as a chronic macrocytic anaemia and there may be a tendency to ‘leave well enough alone’ in an older patient with multiple comorbidities. However, our understanding of MDS continues to improve and better treatment strategies have been developed which can prolong life and also delay transformation to acute leukaemia. Therefore, it is important to evaluate carefully any cytopenia in older patients.
Epidemiology and Clinical Presentation
The exact incidence of de novo MDS remains unclear but appears to be greater than the incidence of acute myelogenous leukaemia (AML).7 In a recent analysis of the SEER database from 2001 to 2004, the age-adjusted incidence rate was 3.3 per 100 000.8 A sharp increase in the incidence of MDS was observed with age: for those aged 80 years and older the incidence was five times that of those aged 60–69 years (35.5 compared with 7.1 per 100 000).8 The age-adjusted incidence of MDS is slightly higher among males than females. However, these are likely underestimations of the true incidence of this disease owing to the underdiagnosis of MDS in the past. The incidence rate appears to have increased from 3.3 per 100 000 per year in 2001 to 3.56 per 100 000 in 2004.9 This increase is probably due in part to an increasing population of people over age 65 years, and also increased recognition and diagnosis of the disease by haematologists and oncologists with the development of more effective therapies.
There are several well-known risk factors for the development of MDS, including exposure to organic solvents, ionizing radiation and prior chemotherapy. The incidence of therapy-related myelodysplasia is also increasing. Therapy-related MDS can result from prior exposure to ionizing radiation or chemotherapy. Prior exposure to alkylating agents is associated with the highest risk of MDS and has very distinct cytogenetic abnormalities, such as loss of the long arm of chromosomes 5 and/or 7. The risk for developing therapy-related MDS or AML has been described in long-term survivors of cancers treated with semustine (methyl-CCNU). The actuarial risk is between 6 and 9% in patients treated for Hodgkin’s disease and 17% in patients treated for multiple myeloma. The risk of developing MDS increases with the use of regional radiation therapy following treatment for common cancers such as breast cancer, small cell lung cancer and testicular cancer. Topoisomerase II inhibitors, such as epipodophyllotoxins and anthracyclines, have also been associated with therapy-related AML, but usually without an MDS prodrome, and commonly involve chromosomal abnormalities such as 11q23, 3q26 and 21q22.10
The clinical presentation of MDS is varied. Symptoms for the most part are non-specific and depend upon the number and severity of cytopenias present. The majority of patients with MDS have a macrocytic anaemia with or without additional cytopenias. Patients with anaemia may have profound symptoms of dyspnoea on exertion, fatigue, lethargy, malaise, dizziness and even angina.11 Neutropenic patients may develop severe systemic infections and infection represents a primary cause of death in many cases of MDS.12 Increased infectious risk may be a result of neutrophil dysfunction secondary to impaired chemotaxis and/or microbial killing.12, 13 T-Cell function is thought to remain intact and therefore patients with MDS less commonly develop viral or mycobacterial infections in the absence of treatment with immunosuppressive agents.12 Other symptoms may include easy bruising or bleeding, a common manifestation of thrombocytopenia and dysfunctional platelets.
The physical findings of MDS are likewise non-specific and usually reflect underlying cytopenias if present. Of note, patients with chronic myelomonocytic leukaemia (CMML) may have splenomegaly, an unusual finding in patients with other subtypes of MDS.
Diagnosis
Blood and Bone Marrow Examination
MDS is diagnosed in patients with one or more cytopenias and depends upon the finding of dysplastic features within the bone marrow in one or more lineages. A peripheral blood smear is helpful in demonstrating a normocytic or a macrocytic anaemia, but alone is insufficient for diagnosis. In order to make the diagnosis, a bone marrow aspirate and biopsy are required. An aspirate is necessary to evaluate morphology, quantify the number of myeloblasts, and assess for cytogenetic abnormalities. A core bone marrow biopsy is used to assess the bone marrow cellularity, which is typically hypercellular and indicative of ineffective haematopoiesis in the setting of peripheral cytopenias.
The morphological features of red cell precursors in the bone marrow aspirate include megaloblastic (asynchronous maturation of the nucleus and cytoplasm) or binucleate or multinucleated cells. Ring sideroblasts may also be identified. These are red cell precursors with iron-laden mitochondria and are defined by the presence of five or more Prussian Blue-staining iron granules encircling more than one-third of the nucleus in more that 15% of the erythroblasts. Erythroid hyperplasia may also be prominent and is associated with ineffective erythropoiesis (a hallmark of MDS).
Abnormalities in the myeloid series can include a left shift with a predominance of immature myeloid cells, hypogranulation and hypolobulation of the nucleus in mature granulocytes. A classic finding is the presence of pseudo-Pelger–Huet cells, which are granulocytes with a bilobed nucleus in a pince-nez configuration. A typical feature in the bone marrow of MDS patients is the presence of >5% myeloblasts. The proportion of myeloblasts has both diagnostic and prognostic information (see below) and is important in differentiating AML from MDS.
The megakaryocytes may likewise be dysplastic and may have not only a quantitative but also a qualitative defect. Examination of the peripheral smear may reveal giant or agranular platelets. In the bone marrow, the megakaryocytes may be small and hypolobulated.
Dysplasia in the bone marrow is not sufficient to establish the diagnosis of myelodysplasia. Deficiencies of vitamin B12 and folate, hypothyroidism, viral infections such as Epstein–Barr and the human immunodeficiency virus and exposure to antibiotics and other chemicals such as ethanol, chemotherapy and benzene can result in dysplasia. These other causes must be ruled out systematically by a careful history and physical and laboratory examination.
Cytogenetics
A critical component of the bone marrow aspiration is the cytogenetic examination of the bone marrow, which not only may help to establish the diagnosis, but also yields important prognostic information (see below). Roughly 60% of patients with MDS have a normal karyotype, but the presence of a common cytogenetic abnormality may establish the diagnosis in difficult cases.14 In addition, new cytogenetic abnormalities may document an evolution to a more clinically aggressive state (i.e. transformation to acute leukaemia). The sensitivity of cytogenetic analysis has been greatly increased by the utilization of fluorescent in situ hybridization (FISH), which uses specific DNA probes to identify rapidly individual chromosomes in hundreds of cells and does not depend upon cell division. The drawback of this approach is that the analysis is restricted to already known and well-established cytogenetic abnormalities.
One series found that cytogenetic abnormalities were more common in the advanced stages of MDS compared with the less advanced MDS subtypes.15 The more common abnormalities are trisomy 8 and deletions of the long arms of chromosomes 5, 7, 11, 13 and 20. Complex karyotypes, defined as three or more cytogenetic abnormalities, are found in 15% of cases and confer a poor prognosis. 15, 16 A sole abnormality involving deletion of 5q is seen commonly in patients with refractory anaemia and represents a distinct clinical syndrome, the ‘5q syndrome’. This syndrome, seen most often in elderly women, is characterized by a prolonged clinical course, which does not typically progress to acute leukaemia. The anaemia is typically profound, but neutropenia is usually mild and platelets are typically elevated. It must be understood that a normal karyotype does not exclude the diagnosis of MDS and is seen in approximately half of the cases of MDS.
Therapy-related MDS is also associated with specific chromosomal abnormalities. In particular, partial or complete loss of chromosome 5 or 7 have been seen after exposure to alkylator therapy, and patients exposed to topoisomerase II inhibitors typically present with a monocytic leukaemia without antecedent MDS and typically have rearrangements of the mixed lineage leukaemia gene located on 11q23.17
Other Genetic Events
Over the past few years, our understanding of the genetic basis for MDS has expanded significantly. Pivotal work by Ebert et al. identified that haploinsufficicny of the ribosomal gene RPS14 cooperates with the loss of micro-RNAs miR-145 and miR-146 which result in key features of the 5q phenotype.18 Mutations in the Ten-Eleven Translocation-2 gene (TET2) were recently identified in MDS and other myeloid neoplasms.19–21 TET2 mutations occur in 10–26% of MDS patients. The enzyme converts methylcytosine to hydroxymethylcytosine and thus may play a role in DNA methylation and may predict response to hypomethylating agents.22 Other recurrent mutations in RUNX1, ASXL1 and TP53 have also been discovered in a substantial proportion of MDS cases. Active investigation is ongoing to determine which of these mutations are causative in the pathogenesis of MDS and which play a role progression of the disease and or may be therapeutically relevant to predict response to therapy.
Classification
The current term MDS was adopted by the French, American and British (FAB) Cooperative Group in 1976 in their classification scheme of these disorders. The WHO classification was proposed as a modification of the FAB system.23 Notably, the criterion for AML was lowered from 30% blasts in the bone marrow to 20% blasts, which eliminated the category of RAEB-t of the FAB classification. Patients with refractory anaemia (RA) with or without ring sideroblasts were divided based upon the presence of unilineage or multilineage dysplasia. Multilineage dysplasia has been shown to impart an inferior prognosis. The category of refractory anaemia with excess blasts was divided into two categories based upon the percentage of blasts. Furthermore, in this classification system MDS associated with a del(5q) was assigned its own category and is associated with a prolonged life expectancy. A new category, MDS-unclassifiable, was introduced to incorporate patients with significant unilineage dysplasia without meeting other criteria for the diagnosis. In addition, chronic myelomonocytic leukaemia has been removed from the MDS category and placed within the myeloproliferative neoplasms category. The WHO was updated in 2008 (Table 32.1).24 Important changes include three categories of refractory cytopenia with unilineage dysplasia, RA, refractory neutropenia (RN) and refractory thrombocytopenia (RT), which reduced the number of cases referred to as unclassifiable.24
Table 32.1 2008 WHO Classification and Criteria of MDS.
| Type | Peripheral blood | Bone marrow |
| Refractory cytopenias with unilineage dysplasia (RCUD) Refractory anaemia (RA) Refractory neutropenia (RN) Refractory thrombocytopenia (RT) | Unicytopenia or bicytopenia <1% or rare blasts | Unilineage dysplasia >10% of the cells in one myeloid lineage <5% blasts ≤15% of erythroid precursors are ring sideroblasts |
| Refractory anaemia with ring sideroblasts (RARS) | Anaemia No blasts | Erythroid dysplasia only <5% blasts ≥15% ring sideroblasts |
| Refractory cytopenia with multilineage dysplasia (RCMD) | Bi- or pancytopenia No/rare blasts No Auer rods Monocytes <1000 μl−1 | Dysplasia in ≥10 % of cells in two or more myeloid cell lines No Auer rods ±15% ringed sideroblasts <5% blasts |
| RAEB-1 (refractory anaemia with excess blasts-1) | Cytopenias <5% blasts No Auer rods | Uni- or multilineage dysplasia 5–9% blasts No Auer rods |
| RAEB-2 (refractory anaemia with excess blasts-2) | Cytopenias 5–19% blasts Auer rods may be present | Unilineage or multilineage dysplasia 10–19% blasts Auer rods |
| MDS-U (MDS unclassified) | Cytopenias No or rare blasts | Unequivocal dysplasia in <10% of cells in one or more myeloid cell lines when accompanied by a cytogenetic abnormality considered as presumptive evidence for a diagnosis of MDS No Auer rods <5% blasts |
| 5q-Syndrome | Anaemia <5% blasts Platelets normal or increased | Normal to increased megakaryocytes with hypolobulated nuclei No Auer rods <5% blasts Isolated del(5q) |
Adapted from WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, IARC Press, Lyon, 2008.24
Prognosis
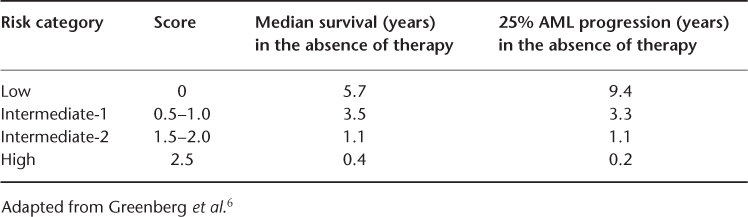
The WHO classification scheme is useful for the diagnostic categorization of patients with MDS, but these diagnostic groups represent heterogeneous patient populations with varied prognoses. Several prognostic systems have been devised to predict the outcome of individual patients better. The most widely used and accepted is the International Prognostic Scoring System, from the International Myelodysplastic Syndrome Risk Analysis Workshop6 (Table 32.2). In this analysis based on over 800 patients, the important predictors for overall prognoses were cytogenetic abnormalities, percentage of myeloblasts in the bone marrow and the number of lineages that exhibited cytopenias. Favourable cytogenetics includes the loss of the Y, 5q or 20q chromosomes or the presence of a normal karyotype. Adverse cytogenetic changes were those with three or more cytogenetic abnormalities or any abnormalities involving chromosome 7. Number scores are attributed to each variable, thus dividing patients into four categories based on the sum of scores for each variable. The median survival for the categories were 5.7, 3.5, 1.2 and 0.4 years for the low-, intermediate-1-, intermediate-2- and high-risk groups, respectively25 (Table 32.3). The time for 25% of the patients in each of the four risk groups to evolve into acute leukaemia was 9.4, 3.3, 1.1 and 0.2 years, respectively. This schema provides very useful prognostic information for patients and counselling regarding treatment options such as haematopoietic stem cell transplantation. However, the usefulness of the IPSS is limited by the fact that patients with secondary MDS were excluded from the analysis and the information only pertains to patients at the time of diagnosis and cannot be used to estimate the real-time risk for patients. Furthermore, it may underestimate the impact of cytogenetics. In this analysis, rare, non-complex cytogenetic abnormalities were placed in the intermediate-risk karyotype group.
Table 32.2 International Prognosis Scoring System (IPSS) in MDS.6, 25.
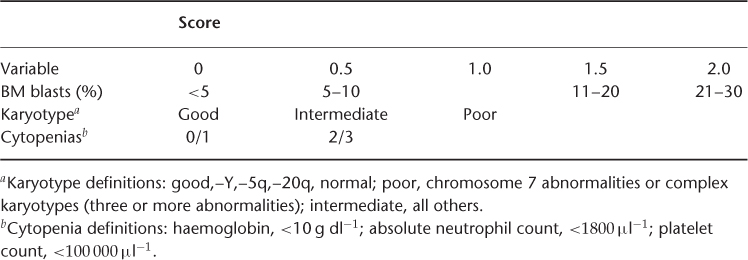
Table 32.3 IPSS Survival for MDS and Evolution to AML.
Haase et al. reviewed the cytogenetic data for over 2124 patients with MDS in Austria and Germany.16 Given the large number of patients, cytogenetic risk groups could be further refined. The median survival was 53.4 months for patients with normal karyotypes and 8.7 months for those with complex anomalies. Thirteen rare abnormalities were identified with good, intermediate and poor prognostic impact (Table 32.4).
Table 32.4 Prognosis of rare cytogenetic abnormalities.
| Risk group | Cytogenetic abnormality |
| Good-prognosis cytogenetics | del(9q) +1/+1q t(1q) t(7q) del(12p) Chromosome 15 anomalies t(17q) Monosomy 21 Trisomy 21 –X |
| Intermediate-prognosis cytogenetics | del(11q) Chromosome 19 |
| Poor-prognosis cytogenetics | Complex, all t(5q), NC 4–6 abnormalities >6 abnormalities |
NC, non-complex Karyotype.
Adapted from Haase et al.16
In an attempt to incorporate dynamic variables such as transfusion burden, the WHO prognostic scoring system (WPSS) was developed.26 This model takes into account WHO subgroups, karyotype and transfusion requirement to classify patients into five risk groups with variable median survivals (12–103 months) and also probability of leukaemia conversion. The advantage of the WPSS is that it is a dynamic prognostic scoring system which can be used for patients at any time during the course of their disease. It has been externally validated and demonstrated to be very helpful particularly for determining the prognosis of patients with low-risk disease.27
To overcome some of the limitations of the IPSS which excludes patients with t-MDS, proliferative chronic myelomonocytic leukaemia and those who have received prior therapy, a group at the University of Texas M.D. Anderson Cancer Center developed a new prognostic scoring system that included all of these patients.28 This model was able to identify four prognostic groups based upon a prognostic score which included features such as age, performance status, platelet count, haemoglobin, white blood cell count, complex karyotype or chromosome 7 abnormality and prior transfusions.
Treatment
The management of patients with MDS requires consideration of several variables, including comorbidities, patient age, severity of cytopenias and the fact that the older patient population in general do not tolerate or respond to conventional therapy. The treatment for MDS is divided into two categories, high and low intensity. High-intensity treatment in general requires hospitalization and includes intensive chemotherapy and haematopoietic stem cell transplant. In contrast, low-intensity treatments are defined as those which can be performed in an outpatient setting, including haematopoietic growth factors, differentiation agents, low-intensity chemotherapy and transfusion support. Key factors in determining high- versus low-intensity treatment are a patient’s age, performance status and IPSS score. High-intensity therapies are usually considered for patients <60 years old, who have good performance status and who fall into the intermediate-2- or high-risk categories. High-intensity treatment may be considered for patients >60 years old who have good performance status and who are in the higher risk categories, but generally patients >60 years of age are considered for low-intensity therapy. Patients >60 years of age in the low- or intermediate-1-risk category are usually considered for supportive care or low-intensity therapy.
Supportive Care
Transfusion Support and Iron Overload
Supportive care is an integral part of the management of all patients with MDS regardless of risk category. Supportive care includes treatment with antibiotics for infection and transfusion support for anaemia and thrombocytopenia. In order to lessen isoimmunization, viral infections and febrile transfusion reactions, leukoreduced and irradiated products are encouraged. In some cases, patients may receive well over 50 units of packed red blood cells. With each unit of blood containing 250 mg of iron, patients may develop iron overload. High levels of iron may lead to secondary haemachromatosis and its resultant adverse hepatic, pancreatic, gonadal and cardiac effects.29 Iron chelation with deferoxamine30 may be administered to these patients; however, this therapy is difficult. Deferoxamine only chelates ∼25 mg of iron per day, must be administered subcutaneously and can lead to chronic skin irritation and cataracts. Two oral iron chelators are now available, deferiprone and deferasirox. Deferiprone reduced hepatic and cardiac iron content in thalassaemia patients, but its utility in MDS is limited by the risk of agranulocytosis and it is not currently approved in the USA.31 Deferasirox is administered once daily and is approved for secondary iron overload in transfusion-dependent anaemias. It has been tested in MDS and found to decrease serum ferritin significantly at 1 year in heavily transfused MDS patients, but exactly what impact that has on total body iron stores or even survival in MDS is unclear.32 Side effects typically include mild nausea and diarrhoea and careful surveillance of renal and hepatic function should be done as nephrotoxicity and hepatotoxicty have been described. Furthermore, baseline hearing and vision tests are recommended prior to initiating the drug and yearly thereafter because of the risk of auditory and ophthalmological disturbances.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree