Figure 167.1 Chest radiograph of a female adolescent with PCR-proven mycoplasmal pneumonia, demonstrating bilateral infiltrates. (Radiograph courtesy of T. Prescott Atkinson, MD.)
Due to widespread lack of diagnostic services, length of time until results can be obtained, impracticality of obtaining diagnostic specimens, and similarity of clinical syndromes due to different microorganisms, clinicians often do not attempt to obtain a microbiologic diagnosis in mild to moderately ill outpatients suspected of having M. pneumoniae infection and elect to treat empirically. However, if the illness is of sufficient severity to require hospitalization, search for a specific microbiologic etiology is justified. If mycoplasmal respiratory infection is to be confirmed, culture, molecular-based, and/or serologic tests are necessary. Clinical laboratories may offer culture service through a reference laboratory familiar with the complex cultivation requirements of mycoplasmas. Respiratory tract specimens suitable for culture include throat swabs, sputum, tracheal aspirates, bronchial lavage fluid, pleural fluid, or lung biopsy tissue according to the patient’s clinical condition. Care should be taken in specimen collection, inoculation into a suitable transport medium, such as SP4 broth, at bedside whenever possible, and not allowing desiccation. Freezing at −70°C is advised if specimens cannot be transported to the diagnostic laboratory immediately after collection. Growth in culture is slow, requiring ≥3 weeks in some cases. Growth of a glucose-fermenting mycoplasma in SP4 broth and development of microscopic spherical colonies approximately 100 μm in diameter on SP4 agar as shown in Figure 167.2 are presumptive evidence of M. pneumoniae. Since some of the upper respiratory commensal mycoplasmal species may also grow on this medium, it is advisable to confirm the identity of the organisms using the PCR assay. Due to the turnaround time, expense, and limited availability, culture is rarely used for routine diagnosis of M. pneumoniae infection.
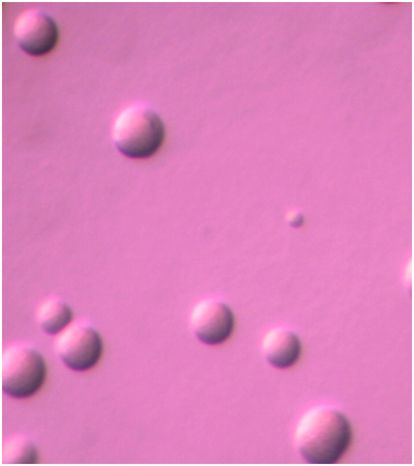
Figure 167.2 Spherical colonies of Mycoplasma pneumoniae approximately 100 μm in diameter growing on SP4 agar. Magnification: 126×.
Serology has been used historically to confirm M. pneumoniae infection. Enzyme-linked immunosorbent assays are preferred over complement fixation assays or cold agglutinin titers. Since primary infection does not guarantee protective immunity against future infections and residual antibody may remain from earlier encounters with the organism, there has been a great impetus to develop sensitive and specific tests that can differentiate between acute or remote infection. Definitive diagnosis requires seroconversion documented by paired serum specimens obtained 2 to 4 weeks apart and assayed at the same time. Although single-titer qualitative and quantitative IgM or IgA assays purported to detect current infection have now become available, IgM can sometimes persist for many weeks after acute infection and as many as 50% of adults may not mount a detectable IgM response. Conversely, some children may not mount a measurable IgG response. Therefore, reliance on a single serologic test can be clinically misleading and paired assays for both IgM and IgG are recommended. Even then, the interpretation of serologic results can sometimes be complex and inconclusive.
Molecular-based systems for rapid detection of M. pneumoniae alone or in combination with other respiratory pathogens are now available through reference laboratories. Some of these assays are commercially sold and others have been developed by individual laboratories for their own use. Given the limitations of culture and serology, molecular-based detection, when available, is the method of choice for detection of acute M. pneumoniae infection.
Treatment of Mycoplasma pneumoniae infections
Formerly it was believed that mycoplasmal respiratory infections were entirely self-limited and no antimicrobial treatment was indicated. More recently it has been shown that appropriate antimicrobial therapy will shorten the symptomatic period and hasten radiologic resolution of pneumonia and recovery, even though organisms may be shed for several weeks. In general, the clinical efficacy of antimicrobial therapy is correlated with severity of pneumonia and elapsed time of illness before treatment is begun. A summary of treatment alternatives for M. pneumoniae respiratory infections is provided in Table 167. 1.
| Drug | Route | Dosage/24 h | Comments | |
|---|---|---|---|---|
| Pediatric | Adult | |||
| Doxycycline | PO | 4 mg/kg loading dose d 1, then 2–4 mg/kg/d in 1–2 doses × 10–14 d | 200 mg loading dose d 1, then 100 mg q12h × 9–13 d | Pregnancy category D Tetracyclines are contraindicated in children under 8 years of age unless there is no other alternative |
| IV | Same as PO | Same as PO | ||
| Tetracycline | PO | 25–50 mg/kg/d in 4 doses × 10–14 d | 250–500 mg q6h × 10–14 d | Pregnancy category D Tetracyclines are contraindicated in children under 8 years of age unless there is no other alternative |
| IV | 10–20 mg/kg/d in 2–4 doses × 10–14 d | 500 mg–1 g q6–12h × 10–14 d | ||
| Erythromycin | PO | 20–50 mg/kg/d in 3–4 doses × 10–14 d | 250–500 mg base/stearate q6h or 400–800 mg ethylsuccinate q6h × 10–14 d | Pregnancy category B |
| IV | 25–40 mg/kg/d in 4 doses × 10–14 d | Same as PO | ||
| Azithromycin | PO | 10 mg/kg/d on d 1, then 5 mg/kg/d × 4 d; not to exceed 250 mg/d | 500 mg d 1, then 250 mg qd × 4 d or 2 g given as a single dose | Pregnancy category B IV formulation is not approved for use in persons under 16 years of age |
| IV | Not recommended | 500 mg qd IV × 2 d, then 500 mg PO qd × 7–10 d | ||
| Clarithromycin | PO | 15 mg/kg/d in 2 doses × 7–14 d | 250–500 mg q12h × 7–14 d (immediate release) or 1 g qd × 7 d (extended release) | Pregnancy category C No IV formulation is available |
| Levofloxacin | PO IV | Not recommended Not recommended | 750 mg qd × 5 d Same as PO | Pregnancy category C Fluoroquinolones are not approved for use in persons under 18 years of age |
| Moxifloxacin | PO IV | Not recommended Not recommended | 400 mg qd × 7–14 d Same as PO | Pregnancy category C Fluoroquinolones are not approved for use in persons under 18 years of age |
| Gemifloxacin | PO | Not recommended | 320 mg qd × 7 d | Pregnancy category C Fluoroquinolones are not approved for use in persons under 18 years of age. No IV formulation is available |
a Treatment recommendations are primarily for management of community-acquired pneumonia with antibiotics approved for treatment of this condition, although they are also appropriate for tracheobronchitis due to this organism. Choice of routes for administration is dependent on the severity of the clinical condition being treated. Most M. pneumoniae infections can be adequately treated with oral medication.
M. pneumoniae is generally susceptible to macrolides, ketolides, tetracyclines, and fluoroquinolones such that in vitro susceptibility testing to guide therapy is not indicated at present. Susceptibility testing would also be impractical most of the time because clinical isolates are generally not available. Macrolide resistance in M. pneumoniae has been known to occur for many years, but it was believed to be quite rare and of minimal clinical significance. Over the past decade, the emergence of macrolide-resistant M. pneumoniae infections in Asia that have resulted in diminished clinical response to macrolide therapy is worrisome. This resistance now affects the majority of M. pneumoniae isolates in China and Japan. Limited data suggest that such resistant organisms also occur in the United States and various countries in Europe, but are still relatively uncommon. Recent data suggest 10% macrolide resistance in the USA. Oral erythromycin has long been a drug of choice for mycoplasmal respiratory infections, but its use has been reduced due to the availability of better-tolerated drugs in this class with improved pharmacokinetics and shorter treatment durations.
The macrolides clarithromycin, azithromycin, and dirithromycin are broad-spectrum agents used primarily for treatment of community-acquired respiratory infections caused by a wide array of bacteria. These agents are very active in vitro against M. pneumoniae and inhibit its growth at comparable or lower minimum inhibitory concentrations (MICs) than those of erythromycin, unless the strain has ribosomal mutations conferring macrolide resistance. All of these drugs have proven clinical efficacy and approved therapeutic indications for pneumonia caused by this organism. Care must be taken because of numerous potential drug interactions with these macrolides. Clarithromycin and azithromycin are available as pediatric oral suspensions and azithromycin is also available as an intravenous formulation in addition to the oral formulations. Tetracycline and its analogs are also effective in vivo and in vitro, but should not be used in children due to potential bone and tooth toxicity. Clindamycin is effective in vitro, but limited reports suggest it may not be active in vivo and should not be considered a first-line treatment. None of the β-lactams, sulfonamides, or trimethoprim is effective in vitro or in vivo against M. pneumoniae.
Fluoroquinolones exhibit bactericidal anti-mycoplasmal activity, but are less potent in vitro than the macrolides against M. pneumoniae. Development of quinolones with documented clinical efficacy and approved indications for treating M. pneumoniae has been driven largely by the need for therapeutic alternatives for β-lactam- and macrolide-resistant S. pneumoniae, and the desire for agents that can be used as empiric monotherapy for respiratory infections due to other typical and atypical organisms. At the present time, quinolones are not approved for use in persons under 18 years of age, but these drugs are rapidly achieving widespread use for treatment of respiratory infections in adults, mainly in the ambulatory setting, and represent reasonable alternative therapies for M. pneumoniae disease, particularly in the setting of macrolide resistance.
Mycoplasmas are slow-growing organisms; thus one would logically expect respiratory infections to respond better to longer treatment courses than might be offered for other types of infections. Thus, a 14- to 21-day course of oral therapy is appropriate for some drugs, but some of the newer agents have shown clinical efficacy against mycoplasmal pneumonias with shorter durations. For example, a 5-day course of oral azithromycin is approved for treatment of community-acquired pneumonia due to M. pneumoniae.
In addition to the administration of antimicrobials for management of M. pneumoniae infections, other measures such as cough suppressants, antipyretics, and analgesics should be given as needed to relieve the headaches and other systemic symptoms. Since most extrapulmonary manifestations are diagnosed late in the course of disease, the benefit of early treatment is unknown. However, limited information suggests that high-dose steroid therapy and/or intravenous immunoglobulin therapy may help reverse neurologic symptoms, if present. If treatment of extrapulmonary mycoplasmal infections is necessary, and/or the patient is immunosuppressed, selection of an agent that exhibits bactericidal activity such as a fluoroquinolone may be most appropriate and administration of the drugs for longer durations may be required.
Fortunately, the treatments of choice for M. pneumoniae are appropriate for many of the other microbial agents responsible for community-acquired respiratory infections. This is especially important in view of the fact that in the major proportion of ambulatory patients seeking medical care, the identity of their infectious organism is never determined.
Genital Mycoplasma and Ureaplasma infections
Ureaplasma spp. and M. hominis can be isolated from the lower genital tract in the majority of sexually active women; their occurrence is somewhat less frequent in men. The presence of genital mycoplasmas in so many asymptomatic persons has made it difficult to prove their pathogenic potential. For some conditions such as cystitis, male urethritis, and urinary calculi for Ureaplasma spp. and pelvic inflammatory disease (PID) for M. hominis
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree