Common mechanisms of gram-negative resistance
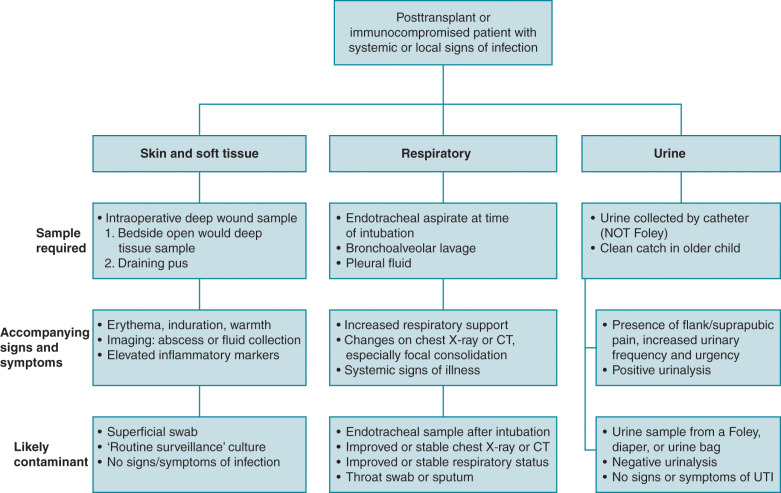
Gram-negative organisms are divided into the Enterobacteriaceae (e.g., Escherichia coli, Klebsiella species, Enterobacter cloacae ) and the glucose nonfermenting gram-negative organisms ( Pseudomonas aeruginosa , Acinetobacter baumannii , Stenotrophomonas maltophilia ). Drug resistance in Enterobacteriaceae is often due to the production of β-lactamases. Common β-lactamases include extended-spectrum β-lactamases (ESBLs), AmpC β-lactamases, and carbapenemases. ESBLs and carbapenemases are generally plasmid mediated, whereas Amp C β-lactamases can either be plasmid mediated or chromosomally mediated.
Common ESBL genes include bla TEM , bla CTX-M-type , and bla SHV , and the associated enzymes result in the hydrolysis of penicillins, cephalosporins, and aztreonam. SHV and the widely spread TEM enzyme were among the first to be recognized from this group; however, CTX-M enzymes are now rapidly becoming a common mechanism of bacterial resistance in many parts of the world. Mechanisms of AmpC β-lactamase resistance in Enterobacteriaceae are divided into three categories: (1) inducible resistance by chromosomally encoded ampC genes (e.g., E. cloacae , Citrobacter freundii, Serratia marcescens ), (2) noninducible chromosomal resistance owing to promoter and/or attenuator mutations (e.g.; E. coli , Shigella spp.); or (3) plasmid-mediated resistance (e.g., E. coli , Klebsiella pneumoniae , Salmonella spp.). Common carbapenemase genes include bla KPC , bla NDM , and bla OXA-48-like . Plasmids that carry ESBL and carbapenemase genes also frequently encode fluoroquinolone resistance, trimethoprim-sulfamethoxazole resistance, and aminoglycoside resistance.
Resistance mechanisms common to P. aeruginosa include OprD porins, hyperproduction of AmpC β-lactamases, upregulation of efflux pumps, and mutations in penicillin-binding proteins, with the minority producing carbapenemases. Drug resistance in Actinobacter baumannii is generally the result of the production of carbapenemases such as OXA-23-like, OXA-40-like, OXA-58-like, and OXA-143-like carbapenemases.
Epidemiology and risk factors
Hematopoietic stem cell transplant recipients.
Patients undergoing allogenic hematopoietic stem cell transplant (HSCT) are at particular risk for invasive infections with gram-negative enteric pathogens. This may be related to conditioning therapy, intestinal graft-versus-host disease, or a prolonged need for parenteral nutrition, all of which can damage the gut epithelium and allow greater translocation of enteric pathogens, resulting in bloodstream infections (BSIs).
A multicenter observational study evaluated the risk factors associated with 848 episodes of multidrug-resistant gram-negative (MDRGN) BSIs in children after HSCT in the United States between 2004 and 2008 and 2011 and 2014. All positive blood culture results between days −10 and +365 surrounding allogenic HSCT were included. Approximately 53% of patients had at least one episode of a gram-negative rod (GNR) BSI; the most frequent pathogens implicated were Klebsiella spp. (26%), Enterobacter spp. (17%), Pseudomonas spp. (16%), E. coli (13%), Stenotrophomonas spp. (7%), and Citrobacter spp. (2%). Approximately 15% of GNR BSIs were caused by organisms resistant to three or more classes of antibiotics. Age older than 16 years and more than one BSI event were risk factors for infection with a resistant organism.
A large study from 65 institutions across 25 countries in Europe, Asia, and Australia was conducted by the European Bone Marrow Transplantation Group to study antibiotic resistance among patients of all ages undergoing autologous or allogenic HSCT. The study results showed that 13% percent of the study participants were 18 years of age or younger. Enterobacteriaceae were significantly more resistant to fluoroquinolones compared with non-Enterobacteriaceae (57% vs. 31%), whereas carbapenem resistance (51% vs. 8%), and multidrug-resistance (47% vs. 32%) was more common in non–lactose-fermenting GNRs, which include P. aeruginosa and A. baumannii. Rates of resistance against all classes of antibiotics were significantly higher in allogenic versus autologous HSCT recipients. In general, GNR resistance in children mirrored proportions in adults, except for fluoroquinolones and antipseudomonal β-lactam/β-lactamase inhibitor combination drugs, for which resistance was higher in adults.
Heart and lung transplant recipients
Infections are a leading cause of death in pediatric heart transplant patients, especially in the first year after transplant, with more than half of infections caused by bacterial pathogens. The Pediatric Heart Transplant Study, a prospectively maintained multiinstitutional research database in the United States, studied bacterial infections in children younger than 18 years less than 1 month, 1 to 6 months and more than 6 months after transplant. Among the GNRs, P. aeruginosa was the most common cause of infection at all time points and was especially more likely to occur in patients with cardiac devices or nosocomial infections. Other important GNRs, regardless of the time point, included Enterobacter spp. (5%), Klebsiella spp. (5%), and E. coli (5%). Data regarding antibiotic resistance were not collected; however, the investigators noted that 2% to 5% of the infections in the first month after transplant were due to organisms commonly resistant to first-line antibiotic agents (e.g., Serratia spp., Citrobacter spp., Stenotrophomonas spp., and Acinetobacter spp.).
Data from a study in adults receiving a solid organ transplant (SOT) showed a steady increase in MDRGN infections (5% in 2007-2008 to 39% in 2014-2015). This was largely driven by an increase in ESBL Enterobacteriaceae. A study in Italy showed that Klebsiella spp., A. baumannii, and P. aeruginosa (49%, 44%, and 31%, respectively) caused the majority of carbapenem-resistant infections. Infection with carbapenem-resistant organisms was more common in patients with a history of lung or heart transplant.
Data are limited in the pediatric lung transplant population to estimate determinants of MDRGN infections. Data from adult lung transplant recipients without underlying cystic fibrosis indicated that P. aeruginosa and Enterobacter spp. were the most common MDRGNs (20% and 19%%, respectively). Any previous exposure to broad-spectrum antibiotics, the presence of a tracheostomy, and an intensive care unit (ICU) stay longer than 14 days were associated with MDRGN bacterial acquisition. Prior colonization with MDRGN bacteria in donors or recipients was not associated with MDRGN infections or worse survival after transplant. Similar results were observed in a cohort of adult cystic fibrosis lung transplant recipients colonized with pan-resistant bacteria, including P. aeruginosa, S. maltophilia, and Burkholderia cepacian .
Liver and intestinal transplant recipients
Children undergoing liver transplants are at high risk of MDGN infections. However, limited data exist regarding the epidemiology of multidrug resistance in this population. In a single-center study of U.S. adults between 2010 and 2014 who received a liver transplant, 53% of bacterial infections were found to be MDR. Among the Enterobacteriaceae, 55% were found to be MDR and 82% were resistant to antibiotics that were used for bacterial prophylaxis.
Among children undergoing intestinal transplants, a U.S. single-center study of BSIs within the first year after transplant showed that almost 24% of the Klebsiella spp. were MDR. Similarly, another U.S.-based study including both adults and children showed that despite use of prophylactic therapy based on individual bacterial resistance patterns, 45% of subsequent infectious episodes were due to MDR organisms. A Spanish study of both children and adults who received an intestinal transplant showed that the most common GNRs isolated were P. aeruginosa, E. coli, and A. baumanni , with 65%, 50% and 100% of respective isolates being MDR.
Renal transplant recipients
A systematic review including both pediatric and adult studies showed that 10% of renal transplant recipients develop urinary tract infections (UTIs) with ESBL-producing Enterobacteriaceae . In a Canadian study enrolling adult renal transplant patients, most UTIs were caused by antibiotic-resistant E. coli or K. pneumoniae ; 5% of isolates were ESBL-producing. Furthermore, resistance to trimethoprim-sulfamethoxazole or fluoroquinolones occurred in 52% and 21%, respectively, of isolated microorganisms. Another study in adult renal transplant recipients showed that although infection with carbapenem-resistant Enterobacteriaceae (CRE) occurred in only 1% of patients, it was associated with higher mortality (30% vs. 10%) and a higher rate of recurrence (50% vs. 22%) compared with patients with more susceptible isolates.
Oncology patients
Approximately one-third of all BSIs in pediatric oncology patients are caused by gram-negative bacteria. A large multicenter study that included both pediatric and adult hospitals in Europe reported that at individual institutions, ESBL-producing bacteria represented 15% to 24% of infections, and carbapenem-resistant P. aeruginosa caused 5% to 14% of infections. Pediatric data from the United States are lacking, but a case-control study in adults was conducted in two New York oncology hospitals to determine the risk factors associated with carbapenem-resistant CRE between 2008 and 2012. Overall, 2% of all BSIs and 5% of all GNR BSIs were caused by CRE. The majority (68%) of CRE organism were K. pneumoniae .
Clinical manifestations of MDRGN infection
Although MDRGN infections in immunocompromised patients occur frequently, clinical manifestations and sites of infection vary widely. Previously noted risk factors for MDRGN infection are shared across all patients, including long hospital stays, ICU admission and ventilation, indwelling catheters and drains, and frequent antibiotic exposure. Prior colonization with MDRGN is also common. Specific sites of infection and clinical disease are often dependent on the method of immune suppression and modifying medical and surgical factors. Although pediatric data are often limited, general lessons drawn from adult literature can be applied to these patients as well.
Solid organ transplant patients
Recipients of SOTs vary in their typical sites of infection by graft type. Many of the pediatric conditions that lead to transplant—intestinal insufficiency, hepatic failure, congenital heart disease, cystic fibrosis—require intensive inpatient care, which can lead to a significant risk for infection before receipt of the graft. The complex postsurgical care needs often involve catheter placement and risk for catheter-related infection and associated bacteremia, whereas mechanical ventilation puts many recipients at risk of ventilator-associated pneumonia. Specific graft types also lead to increased incidence of specific clinical syndromes. In renal transplant recipients, both UTIs and surgical site infections with MDRGN develop. For kidney transplant recipients, ESBL-producing E. coli accounts for up to 12% of infection, especially in high-risk adult patients. Approximately 70% of the complications caused by ESBL-producing GNR are UTIs. In contrast, liver transplantation requires extensive abdominal surgical procedures and posttransplant infections reflect this. A 2008 survey of pediatric liver transplant recipients noted that 38% of patients have bacterial infection in the first year after transplant, composed of central line (39%), intraabdominal (35%), wound (14%), and biliary infections (7%). Gram-negative infections predominate in all these sites, and the overall prevalence of MDRGN among these infections was recently estimated at 7%, although for bacterial infection, respiratory tree syndromes predominate. A recent survey of pediatric lung transplantation noted a 22% rate of postoperative infection. Whereas bacteremia predominated in the first month postoperatively, respiratory tract infection with MDRGN was seen more commonly after that period. In this study, prior history of MDRGN, especially in the setting of cystic fibrosis, led to elevated overall risk of infection. Infections in pediatric heart transplant are dominated by bacteremia, pneumonia, and surgical site infections. Recent data show that although BSIs (25%) are more common than pneumonia (21%), MDRGN infections are more commonly associated with respiratory infection.
Oncology and hematopoietic stem cell transplant patients
Regardless of type of malignancy or HSCT, oncology patients all share the risks for MDRGN infection similar to other chronically hospitalized patients. The presence of indwelling catheters, exposure to immune suppression and mucositis risk, and colonization with prior pathogens all increase this risk. Patients in a large prior survey of infections in children with cancer found that bloodstream (29%), pulmonary (18%), and skin and soft tissue (15%) are the most common sites in pediatric patients. Among these, bacteremia associated with mucositis and catheter infections is the most common MDRGN infection found in oncology patients.
Disease prophylaxis and prevention
Decolonization practices and benefits for MDRGN disease are less well established than for gram-positive pathogens. Decontamination of the respiratory and digestive trees is based on presumed reduction of infection risk by altering the biome to reduce the burden of MDRGN in these sites. Methods include oropharyngeal decontamination with antiseptic agents and selective digestive tract decontamination with nonabsorbable antibiotics. Prior studies have indicated that selective oral and digestive tract decontamination reduces rates of bacteremia and mortality in ICU patients, although the overall benefit appears modest and conflicting data exist. Use in U.S. hospitals has been limited by concerns for induction or worsening of antibiotic-resistant pathogens, as initial studies in the European centers were conducted in hospitals with low rates of antibiotic resistance. These differences in incidence limit the generalizability of findings for areas more endemic for MDRGN resistance. Oral decontamination regimens typically use chlorhexidine (CHG) gluconate, an antiseptic with broad-spectrum bacterial activity, including MDRGN. Daily CHG bathing has been shown to decrease CRE skin colonization, and large-scale studies have shown benefit of CHG bathing in reducing the risk of hospital-acquired BSIs. Although studies specifically focusing on the role of CHG bathing in preventing MDRGN transmission have not been conducted, various bundles that have been successful in reducing MDR GNR transmission have included daily 2% CHG bathing. Concerns of gram-negative microorganisms developing resistance to CHG have tempered some of the enthusiasm for it, although some recent data suggest this may not be a drawback.
Screening for colonization of SOT recipients with MDRGN is generally recommended in the pretransplant period. As noted previously, risk factors for MDRGN infection include colonization in the pretransplant period, which has been linked to infection after graft placement. Despite the strong connection between colonization and infectious complications, no clear data exist regarding the effectiveness of perioperative prophylactic regimens in children. Screening of potential donors specifically for MDRGN colonization is not recommended by any major society, although targeted therapy to treat donor-derived infections is considered on a case-by-case basis. American Society for Transplantation guidelines for recipients who are ESBL carriers are limited to specific prophylaxis not exceeding 48 hours of duration, with the exception of lung transplant recipients for whom longer courses are acceptable. Specific recommendations for other MDRGN do not exist except for standard surgical prophylaxis.
Oncology patients and bone marrow transplant recipients are at high risk for MDRGN infections. One series documented that isolation of MDRGN from any site in the prior 12 months increased the risk for MDRGN bacteremia almost 9-fold. However, no consensus guidelines beyond those noted in the aforementioned recommendations exist for pretransplant screening or ongoing screening of recipients for MDRGN colonization at specific sites. Although routine use of levofloxacin prophylaxis in high-risk patients undergoing chemotherapy and bone marrow transplant has increased in frequency, some data suggest an increase in development of resistant GNR in response to this approach. Modification of levofloxacin prophylaxis in selected oncology patients with a prior history of MDRGN has not been extensively studied and thus no definitive recommendations exist for this clinical scenario in either pediatric or adult patients.
Diagnosis
With limited options for the treatment of MDRGN infections, it is essential to adequately differentiate true invasive infection from colonization. Bacterial cultures from endotracheal aspirates and urine in the absence of appropriate signs and symptoms suggestive of infection may lead to the unnecessary overuse of antibiotics and associated downstream consequences. Fig. 14.1 provides an overview of clinical signs and symptoms to assist with determining if a bacterial isolate is likely a pathogen or a contaminant when isolated from nonsterile sites.