Head and neck cancer is a heterogeneous group of cancers, which require a multidisciplinary approach to achieve excellent treatment results. This article focuses on current treatment guidelines and controversies in the management of head and neck cancer. It also provides insight into future directions and newest advances in the treatment of head and neck cancer.
Key points
- •
Squamous carcinoma of the head and neck cancer is the sixth most common cancer in the United States, with an increasing incidence largely because of a rising epidemic of human papilloma virus (HPV)-positive oropharyngeal cancer.
- •
Early-stage or locally advanced head and neck cancer is treated with curative intent, and management is a multidisciplinary effort, which should encompass head and neck surgeons, radiation oncologists, and medical oncologists.
- •
Metastatic head and neck cancer is treated with palliative intent with platinum-based or taxane-based chemotherapy regimens, such as cisplatin/5-fluorouracil and cisplatin/paclitaxel. The addition of cetuximab to this regimen has been the only progress over the past decade that has shown an increase in overall survival.
- •
HPV-positive head and neck cancer has a distinct pathogenesis compared with the classic HPV-negative, smoking/alcohol-induced head and neck cancer, which is caused by a stepwise accumulation of multiple mutations. HPV-positive cancers typically have a better prognosis compared with HPV-negative cancers.
- •
Head and neck cancer is a heterogeneous disease with distinct genetic subsets, which are under investigation.
Introduction
Head and neck cancer (HNC) comprises a heterogeneous group of cancers, which primarily affect the oral cavity, oropharynx, hypopharynx, larynx, and nasopharynx. In 2010, HNC was the sixth most frequent cancer by incidence worldwide, with more than 500,000 new cases diagnosed. The highest incidence rates occur in South-Central Asia and Central and Eastern Europe. In North America and Europe, oral cavity, oropharyngeal, and laryngeal cancer are the most common types, whereas nasopharyngeal cancer (NPC) is more common in the Far East and Mediterranean countries.
Classic HNC is caused by smoking and alcohol exposure, which drive a stepwise accumulation of mutations in the squamous epithelium ( Box 1 ). One of the most important developments in HNC is the discovery that human papilloma virus (HPV) subtype 16 is the causative factor in most oropharyngeal cancers (base of tongue, tonsil), especially in North America and Western Europe. Based on several retrospective studies, 40% to 60% of patients with oropharyngeal cancer in the United States are HPV-positive. As the incidence of smoking has decreased, the incidence of HPV-negative HNC has also declined, whereas the rates of HPV-positive HNC have increased, accounting for the rising incidence of HNC. These cancers are usually seen in young, nonsmoker, nondrinker men, and they have a better prognosis compared with HPV-negative HNC. The pathogenesis of these cancers has been attributed to 2 viral proteins, E6 and E7, which bind and inactivate 2 tumor suppressor genes, p53 and retinoblastoma Rb, respectively. This process drives malignant transformation of the squamous epithelium. Based on these data, HNC is now classified into HPV-positive and HPV-negative HNC. Another important viral cause of HNC is the Epstein-Barr virus (EBV), which is associated with the endemic form of NPC in Asia and Africa. Box 2 summarizes the major risk factors in the pathogenesis of HNC.
- •
Mutations
- ○
TP53
- ○
CDKN2A (p16)
- ○
PIK3CA
- ○
NOTCH1
- ○
- •
Copy number alterations
- ○
EGFR
- ○
VEGF1
- ○
CCND1
- ○
MYC
- ○
CTTN
- ○
- •
Chromosomal deletions/gains
- ○
3p, 3q, 7p, 7q, 8q, 9p, 10q, 11q, 17p, 18q
- ○
- •
Smoking
- •
Alcohol (>50 g/d)
- •
Previous history of HNC
- •
Advanced age (>50 years)
- •
Hereditary predisposition (rare):
- ○
Fanconi anemia
- ○
- •
Viral agents:
- ○
HPV (oropharyngeal cancer)
- ○
EBV (NPC)
- ○
Clinically, a precursor premalignant lesion may precede HNC, such as oral leukoplakia or erythroplakia in the cheeks, gums, or tongue. Symptoms include mouth, throat, neck, and ear pain, persistent mouth sores, hoarseness, persistent unilateral nose bleeding or nasal obstruction, unilateral hearing loss, dysphagia, odynophagia, a persistent palpable neck mass and cranial neuropathies, especially with NPC. They usually spread by invasion of adjacent local structures and regional lymph nodes. Systemic metastasis is rare at presentation (<10%). Patients may also have other synchronous multiple primary malignancies or metachronous malignancies, such as second primary HNC, lung, or esophageal cancer, which occur in 20% of survivors. This finding reflects the exposure of the upper aerodigestive mucosa to the same carcinogens, an effect known as field cancerization.
Diagnosis is made by triple endoscopy (laryngoscopy, esophagoscopy, and bronchoscopy), which also allows for investigation of synchronous malignancies. Complete staging includes computed tomography (CT) or magnetic resonance imaging (MRI) of the head and neck, and CT of the chest to rule out pulmonary metastasis, although these are rare.
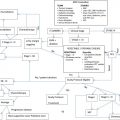
The treatment of HNC requires a highly specialized, multidisciplinary team ( Box 3 ) and should ideally take place in centers of excellence in the management of this disease. This article focuses on the mainstay approaches and most recent advances in the multidisciplinary treatment of patients with HNC. The emerging role of HPV, molecular profiling, targeted therapy, and immunotherapy are also briefly reviewed.
- •
Medical oncology
- •
Head and neck surgery
- •
Radiation oncology
- •
Plastic and reconstructive surgery
- •
Palliative medicine
- •
Psychiatry
- •
Ancillary services
- ○
Nutrition
- ○
Social work services
- ○
Tracheostomy care
- ○
Speech and swallow evaluation/therapy
- ○
Smoking and alcohol cessation counselling
- ○
Dental care before chemoradiation
- ○
Introduction
Head and neck cancer (HNC) comprises a heterogeneous group of cancers, which primarily affect the oral cavity, oropharynx, hypopharynx, larynx, and nasopharynx. In 2010, HNC was the sixth most frequent cancer by incidence worldwide, with more than 500,000 new cases diagnosed. The highest incidence rates occur in South-Central Asia and Central and Eastern Europe. In North America and Europe, oral cavity, oropharyngeal, and laryngeal cancer are the most common types, whereas nasopharyngeal cancer (NPC) is more common in the Far East and Mediterranean countries.
Classic HNC is caused by smoking and alcohol exposure, which drive a stepwise accumulation of mutations in the squamous epithelium ( Box 1 ). One of the most important developments in HNC is the discovery that human papilloma virus (HPV) subtype 16 is the causative factor in most oropharyngeal cancers (base of tongue, tonsil), especially in North America and Western Europe. Based on several retrospective studies, 40% to 60% of patients with oropharyngeal cancer in the United States are HPV-positive. As the incidence of smoking has decreased, the incidence of HPV-negative HNC has also declined, whereas the rates of HPV-positive HNC have increased, accounting for the rising incidence of HNC. These cancers are usually seen in young, nonsmoker, nondrinker men, and they have a better prognosis compared with HPV-negative HNC. The pathogenesis of these cancers has been attributed to 2 viral proteins, E6 and E7, which bind and inactivate 2 tumor suppressor genes, p53 and retinoblastoma Rb, respectively. This process drives malignant transformation of the squamous epithelium. Based on these data, HNC is now classified into HPV-positive and HPV-negative HNC. Another important viral cause of HNC is the Epstein-Barr virus (EBV), which is associated with the endemic form of NPC in Asia and Africa. Box 2 summarizes the major risk factors in the pathogenesis of HNC.
- •
Mutations
- ○
TP53
- ○
CDKN2A (p16)
- ○
PIK3CA
- ○
NOTCH1
- ○
- •
Copy number alterations
- ○
EGFR
- ○
VEGF1
- ○
CCND1
- ○
MYC
- ○
CTTN
- ○
- •
Chromosomal deletions/gains
- ○
3p, 3q, 7p, 7q, 8q, 9p, 10q, 11q, 17p, 18q
- ○
- •
Smoking
- •
Alcohol (>50 g/d)
- •
Previous history of HNC
- •
Advanced age (>50 years)
- •
Hereditary predisposition (rare):
- ○
Fanconi anemia
- ○
- •
Viral agents:
- ○
HPV (oropharyngeal cancer)
- ○
EBV (NPC)
- ○
Clinically, a precursor premalignant lesion may precede HNC, such as oral leukoplakia or erythroplakia in the cheeks, gums, or tongue. Symptoms include mouth, throat, neck, and ear pain, persistent mouth sores, hoarseness, persistent unilateral nose bleeding or nasal obstruction, unilateral hearing loss, dysphagia, odynophagia, a persistent palpable neck mass and cranial neuropathies, especially with NPC. They usually spread by invasion of adjacent local structures and regional lymph nodes. Systemic metastasis is rare at presentation (<10%). Patients may also have other synchronous multiple primary malignancies or metachronous malignancies, such as second primary HNC, lung, or esophageal cancer, which occur in 20% of survivors. This finding reflects the exposure of the upper aerodigestive mucosa to the same carcinogens, an effect known as field cancerization.
Diagnosis is made by triple endoscopy (laryngoscopy, esophagoscopy, and bronchoscopy), which also allows for investigation of synchronous malignancies. Complete staging includes computed tomography (CT) or magnetic resonance imaging (MRI) of the head and neck, and CT of the chest to rule out pulmonary metastasis, although these are rare.
The treatment of HNC requires a highly specialized, multidisciplinary team ( Box 3 ) and should ideally take place in centers of excellence in the management of this disease. This article focuses on the mainstay approaches and most recent advances in the multidisciplinary treatment of patients with HNC. The emerging role of HPV, molecular profiling, targeted therapy, and immunotherapy are also briefly reviewed.
- •
Medical oncology
- •
Head and neck surgery
- •
Radiation oncology
- •
Plastic and reconstructive surgery
- •
Palliative medicine
- •
Psychiatry
- •
Ancillary services
- ○
Nutrition
- ○
Social work services
- ○
Tracheostomy care
- ○
Speech and swallow evaluation/therapy
- ○
Smoking and alcohol cessation counselling
- ○
Dental care before chemoradiation
- ○
Principles of multimodality therapy in HNC
Ninety percent of patients with HNC present with early-stage (I and II) or locally advanced disease (stage III, IVA, IVB), whereas 10% of patients have metastatic (stage IVC) disease. Almost 50% of patients with HNC can be cured. Patients with early-stage disease can be cured in 80% to 90% of cases with single modality therapy, either radiotherapy (RT) or surgery. On the other hand, patients with locally advanced disease require a multimodality approach with surgery, RT, and chemotherapy and achieve cure rates of 40% to 50%. Recurrent or metastatic disease is incurable and managed with systemic chemotherapy, although 15% of patients with locally recurrent disease may have reirradiation or salvage surgery.
Based on stage, HNC can be resectable or unresectable. However, even if a tumor is curable with resection, surgery may confer significant morbidity and compromise in quality of life, because of the anatomic complexity of the head and neck structures, most importantly the nasopharynx, oropharynx, hypopharynx, and larynx. In these cases, an organ-preservation approach is favored, with RT or concurrent chemoradiotherapy (CRT). In a similar philosophy, although early-stage cancers of the oral or nasal cavity can be cured with surgery without significant functional impairment, a nonsurgical strategy with CRT is still possible with equivalent cure rates. This approach is known as surgical sparing.
Mitigation of side effects by surgery, RT, and chemotherapy is of paramount importance to avoid acute and chronic complications. Surgical techniques are evolving toward less invasive approaches, whereas reconstructive procedures are used to achieve a better functional and aesthetic outcome. Radiation therapy techniques are gravitating toward fractionation schedules and intensity-modulated radiation therapy, which focuses on the organ of interest and spares surrounding healthy tissues. Although chemotherapy remains an integral part of a multimodality strategy, research efforts are focusing on the identification of targeted agents, which could be equally or more efficacious and at the same time less toxic.
Principles of surgery
Introduction
Treatment of HNC is a careful balance between morbidity and cure. Whereas traditional open surgical approaches remain the gold standard for many advanced tumors, the last decade has seen an increasing focus on minimally invasive approaches for early and select advanced tumors. Advances in endoscopic, robotic, and laser technology have allowed use of natural orifices and have increased the feasibility of large tumor extirpation. Adoption of sentinel lymph node mapping has also begun to occur, signaling a new era of minimal neck dissections.
In addition to serving as a primary modality, surgery is an important salvage modality after RT or CRT at both the primary site and the neck. Open and even minimally invasive approaches are feasible in a previously irradiated patient, but typically with decreased functional outcomes and higher complication rates. Free flap reconstruction is commonly used in these patients and is an important part of the head and neck surgeon’s armamentarium.
Transoral, Minimally Invasive Approaches
Minimally invasive or natural orifice surgery is a natural extension of the endoscopic diagnostic techniques that have been developed over decades of head and neck surgery. These approaches have usefulness for transnasal extirpation of skull base and sinus malignancies and transoral resection of laryngeal and oropharyngeal tumors. The art of the latter approaches extends beyond the elimination of external scars and lies in the maintenance of the physiologic muscular layers of the swallowing mechanism and the preservation of respiratory and phonatory function.
Minimally invasive conservation surgery for laryngeal cancer is well established for both early-stage and late-stage tumors. Local control rates for T1 tumors are reported to be 91% to 99%, similar to results of RT. Local control is worse with anterior commissure involvement for both modalities. For T2 tumors, there is low-level evidence to suggest that surgery may offer a better local control and a higher rate of laryngeal preservation, but definitive studies have not been conducted. For T3 laryngeal disease, reports of transoral laser and open conservation surgery combined with adjuvant CRT show laryngeal preservation rates in the 80% to 90% range. Much like with CRT, patients after surgery for advanced laryngeal cancer should expect some degree of aspiration and must have appropriate lung reserve to clear secretions.
For oropharyngeal cancers, minimally invasive surgery plus neck dissection has been shown in prospective cohorts to have oncologic efficacy outcomes comparable with CRT for both HPV-positive and HPV-negative tumors, although comparative efficacy studies are lacking. The oncologic principles of both transoral laser microsurgery (TLM) and transoral robotic surgery (TORS) are the same: precise, complete, microscopic excision of expendable portions of the pharynx through a natural orifice. For both techniques, patients also undergo neck dissection with or without postoperative adjuvant therapy based on pathologic staging. The principle difference between these techniques is that TLM relies on a microscope and laser, whereas TORS relies on the DaVinci Surgical System (Intuitive Corporation, Sunnyvale, CA). Adoption of and publications regarding TORS have increased at a faster rate than for TLM, presumably because of enthusiasm for robotic technology. However, both techniques report similar oncologic results.
Success with TLM with or without adjuvant CRT for treatment of oropharyngeal cancer was first reported by Steiner in 2003, with a report of 48 patients with mainly locally advanced base-of-tongue cancer, showing a 5-year local control rate of 85%. These data were reinforced by a multicenter study of 204 patients with advanced oropharyngeal cancer treated with TLM, with a 2-year and 5-year survival rate of 89% and 78%, respectively. In one-quarter of the patients, adjuvant therapy was avoided altogether, and only 16% required combined CRT.
Functional outcomes with TLM have been favorable, with 87% of patients in the multicenter study achieving a normal or near-normal diet at last follow-up. In addition, 83% of patients did not require a gastrostomy tube (G-tube) during surgical therapy and healing. Of the patients without G-tubes, 22% later required gastrostomy during adjuvant therapy, for a total of a 44% temporary G-tube rate. However, at 2 years, this rate was only 9.3% and at 5 years only 3.8%.
TORS for oropharyngeal cancer was first pioneered by Weinstein and O’Malley in 2006 and has been widely adopted. The bulk of the present data on TORS are for oropharyngeal cancer, primarily early T-stage (T1–T2) and often with advanced neck disease. In studies with sufficient follow-up, 2-year overall survival (OS) rate has been reported in the 80% to 95% range.
Similar to TLM, studies of functional outcomes for TORS have reported low G-tube dependency rates of less than 10% at 1 year. It is unclear whether this finding is part of a philosophy of aggressive swallowing therapy and avoidance of prophylactic G-tubes or whether this low dependency rate is a salutary effect of decreased dosing or complete avoidance of RT.
Trials of both TLM and TORS have used postoperative adjuvant therapy based on pathologic staging, and oncologic results must be considered in light of the 70% to 80% rate of postoperative adjuvant therapy in these series. Indications for postoperative CRT are positive or close surgical margins (<2 mm), extracapsular spread in metastatic lymph nodes, and postoperative radiation for multiple metastatic lymph nodes, lymphovascular invasion, and perineural invasion. In these series, the postoperative radiation dosing was approximately 10 Gy lower compared with primary dosing, with the exception of patients with positive margins. The degree to which specific reductions in postoperative RT affect swallowing and other functional and quality-of-life–related outcomes remains unknown.
Surgical Management of the Neck
Neck dissection has evolved from morbid, en-bloc radical neck dissections that remove the sternocleidomastoid muscle, internal jugular vein, and cranial nerve XI to an approach introduced by Bocca of the selective neck dissection, using natural fascial planes to remove the lymphatic tissue but sparing normal structures. This approach necessitates certain features, such as mobile nodes or a clinically negative neck, and encompasses all of the anticipated drainage basins of a given tumor. For instance, neck dissection for oral cancer typically involves levels I to III (supraomohyoid) and for laryngeal cancer involves levels II to IV (lateral), subject to clinical findings and the presence of lymph nodes.
Selective neck dissection can also be used as salvage or to assess persistent nodes in patients who have undergone CRT. In these cases, the dissections can often be even more limited (superselective) based on radiographic workup. CRT can restore radiographic tissue planes and make the neck anatomy more amenable to selective dissection, so operative planning should be based on posttreatment scanning and examination. Routine post-CRT neck dissection has been abandoned and many centers rely on positron emission tomography (PET) scanning for post-CRT assessment of neck response.
Elective neck dissection in the clinically and radiographically N0 neck is performed for patients with significant risk for occult metastases, based on tumor location and depth of invasion. However, there is an inherent trade-off between the morbidity of neck dissection and the risk of subsequent development of neck disease. In patients who are observed and develop neck metastases, the morbidity of neck dissection is higher, and in some series, the success rate is lower. This finding prompted investigation of an alternative approach to the N0 neck using intraoperative mapping of the drainage patterns of head and neck tumors with selective biopsy of the first-echelon (sentinel) nodes.
Sentinel lymph node biopsy (SLNB) in the head and neck was first introduced in 1992 for melanoma but has been expanded in the last decade for evaluation of the clinically and radiographically N0 neck in oral and oropharyngeal squamous carcinoma. The presence of lymph node metastases is the most significant negative prognostic indicator for HNC, and SLNB offers a minimally invasive means of assessing the status of the lymphatic basins. Compared with elective neck dissection, SLNB has been shown to result in better postoperative quality of life and shoulder function, as well as decreased scar size and a lower complication rate.
Several multi-institutional groups have conducted trials of SLNB for the treatment of the N0 neck, with long-term results now becoming available. The ACSOG (American College of Surgeons Oncology Group) trial reported a negative predictive value (NPV) of 96% at 5 years for SLNB with immunohistochemistry evaluation. All patients were determined radiographically N0 by CT or MRI, and all surgeons underwent standardized training. Similarly, a European multicenter trial reported an NPV of 94% at 5 years. However, these investigators found the NPV to be significantly worse for floor-of-mouth tumors (88%) compared with other sites (98%). Most recently, SLNB has been shown to be feasible in patients who have had previous neck surgery or irradiation, with 100% regional control at a median follow-up of 22 months. This approach in previously treated patients holds promise and warrants further study.
Salvage Surgery
For patients who have recurrent or persistent disease after radiation or CRT, salvage surgery is an option for cure or palliation. Nonsmoking patients with early-stage recurrent cancers (rT1–rT2) are better candidates for curative-intent salvage surgery compared with patients with advanced-stage cancers who continue to smoke. Although some patients with smaller defects can be allowed to granulate, which takes considerably longer in an irradiated field, or in a region reconstructed with local flaps, which are more tenuous than in nonirradiated patients, many of these patients require axial or free flap reconstruction.
In a prospective study, the average survival at 5 years for patients undergoing salvage surgery was 39%, and the median disease-free survival was 17.9 months. In this study, higher stage was associated with shorter survival, with median survival for stage I to III of 21 to 24 months and only 9.3 months for stage IV. Patients who recurred sooner (<6 months) after previous treatment (surgery, radiation, or chemotherapy) trended a shorter mean survival of 18.3 months versus more than 21 months for patients with longer times to recurrence. In addition, lower-stage patients also had a better chance of quality-of-life improvement after surgery compared to higher-stage patients (64% for stage I vs 39% for stage IV).
Some patients are rendered cancer free but nonfunctional after CRT. Scarring, loss of sensation, and tumor-related cartilage destruction can all contribute to a suboptimal functional status. Elective laryngectomy is an appropriate option for patients with severe laryngeal dysfunction and aspiration, and has a high success rate of restoring oral intake and improving quality of life. These patients can also have voice restoration with a tracheoesophageal prosthesis, but complications of these prostheses are increased in patients with previous RT.
Reconstruction
Reconstruction is a critical aspect of head and neck surgery. Goals of reconstruction are to ensure a watertight seal of the digestive tract and skull base, maintain function of critical structures (eg, oral sphincter, tongue, jaw, larynx, pharyngoesophagus), and maintain cosmesis. Local flaps derived from the mucosa, nasolabial folds, and neck skin can provide good intraoral lining. Axial flaps such as the pectoralis major myocutaneous and myofascial flaps, the temporalis or temporoparietal fascial flaps, and the supraclavicular artery island flap have multiple applications for reconstruction and are reliable, although limited by their pedicle. Free tissue transfer has become routine in most major medical centers and offers healthy, nonirradiated tissue, with full freedom of inset geometry and multiple tissue types.
Plastic surgeons, and with increasing frequency, head and neck surgeons all participate in reconstructive efforts by institution basis. The most common donor sites are anterolateral thigh (bulk, large skin), radial forearm (thin and pliable skin, bone available), and fibula (longest bone stock, skin). The subscapular system provides a variety of tissue and can be used for multiple applications, but the posterior location of the donor site makes simultaneous tumor resection and flap harvest challenging.
Overall success of microvascular free flap reconstruction of head and neck defects is more than 95%, but a quarter or more of patients can expect some perioperative complication. Previous RT may lower the threshold for use of free flap reconstruction but does not seem to increase the complication rate.
Principles of radiation therapy
Indications
For early-stage HNC, RT can offer an alternative to surgical resection as definitive treatment. For locally advanced disease, most patients are treated either with concurrent CRT or surgery followed by adjuvant RT with or without chemotherapy. Box 4 summarizes the indications for adjuvant RT or CRT for early and locally advanced HNC.
Indications for Adjuvant RT
- •
One lymph node greater than 3 cm
- •
Multiple positive nodes (no extracapsular extension)
- •
Lymphovascular invasion
- •
Perineural invasion
- •
pT3 or pT4 primary tumor
- •
Oral cavity/oropharyngeal primary with positive level IV/V nodes
Indications for Adjuvant CRT
- •
Extracapsular extension
- •
Positive margins
Altered Fractionation Radiation
Standard radiation treatments are usually given in 2-Gy fractions per day, 5 days a week, to a total dose of 60 to 70 Gy. Altered fractionation schemes that take advantage of principles of radiation biology have been investigated to try to improve local control and toxicity rates.
Hyperfractionation
Hyperfractionation involves delivering 2 to 3 fractions per day, usually in a smaller dose per fraction than 2 Gy. Treatment in this fashion is believed to decrease late toxicity by taking advantage of the fact that late-responding normal tissues are more sensitive to large doses per fraction. Therefore, multiple treatments with smaller doses should theoretically decrease late toxicity. Consequently, the total dose of treatment using hyperfractionation can often be escalated without causing unacceptable toxicity levels.
Accelerated fractionation
In accelerated fractionation schemes, the total treatment time is reduced to try to decrease the probability of tumor repopulation during treatment. In pure accelerated fractionation, the total dose and dose per fraction remain the same, but more fractions may be given per week. In hybrid models, the dose is altered.
Some of the main radiation trials involving altered fractionation are listed in Table 1 . In aggregate, these trials suggest a locoregional benefit to altered fractionation radiation. Most of the trials investigating altered fractionation compared with standard fractionation have been performed with radiation alone. The role of altered fractionation in combination with cisplatin is being investigated. In RTOG 0129, 70 Gy in 2-Gy fractions with 3 cycles of cisplatin is being compared with 72 Gy delivered with a delayed concomitant boost (twice-daily radiation for the last 12 days) plus 2 cycles of cisplatin. The preliminary results of this study did not show a survival or locoregional control benefit to altered fractionation compared with the standard fractionation arm.
| Type of RT | Trial | Tumor Type and Stage | N | RT Dose | LCR | OS | Late Toxicity |
|---|---|---|---|---|---|---|---|
| HF | EORTC 22791 (1992) | T2-3N0-1 OPx | 159 166 | 70/1.8–2 Gy 80.5/1.15 Gy twice a day | 5 y 40% 59% ( P = .02) | No difference ( P = .08) | No difference in ≥Gr 2 ( P = .72) |
| HF, AF | RTOG 90-03 (2000) | Stage II–IV (BOT or hypopharynx) Stage II–IV (OC, OPx, and supraglottic larynx) | 268 263 274 268 | 70/2 Gy 81.6/1.2 Gy twice a day 67.2/1.6 Gy twice a day (split course) 72/1.8 Gy + 1.5 Gy DACB | 2 y 46% 54.4% ( P = .045) 47.5% ( P = .55) 54.5% ( P = .05) | 2 y 46.1% 54.5% ( P = .13) 46.2% ( P = .86) 50.9% ( P = .40) | Late ≥grade 3: 26.8% 28%, NS 27.6%, NS 37.2% ( P = .011) |
| HF/AF | EORTC 22851 (1997) | T2-4N0-3 (no hypopharynx) | 253 247 | 70/1.8–2 Gy 72/1.6 Gy three times a day | 5 y 46% 59% ( P = .02) | No difference ( P = .96) | Late ≥grade 3: 4% 14% ( P <.001) |
| AF | CHART (2010) | Locally advanced HNC (no T1N0 tumors) | 552 336 | 54/1.5 Gy three times a day 66/2 Gy | HR = 0.96 ( P = .71) | HR = 1.05 ( P = .62) | Late mucosal necrosis: 5% vs 9% ( P = .02) Grade 3 xerostomia: 23% vs 31% ( P = .02) Laryngeal edema: 50% vs 60% ( P = .05) |
| AF | DAHANCA 6/7 (2003) | Stage I–IV HNC a | 726 750 | 66–68/5 fxn per wk 66–68/6 fxn per wk | 5 y 60% 70% ( P = .0005) | No difference ( P = .78) | Severe late toxicity: no difference ( P = .16) |
Dose and Treatment Volumes
To define the treatment volume, findings on physical examination, examination under anesthesia (if applicable), and multimodality imaging (including CT, MRI, or PET) are used. For definitive RT, the high-dose volume includes the areas of gross disease at the primary site and nodal regions (gross tumor volume [GTV]). The GTV is then expanded based on the anatomy of spread to account for microscopic disease involvement (clinical tumor volume [CTV1]). The CTV1 is expanded by another 2.5 to 10 mm to the planning target volume (PTV1). The typical treatment dose prescribed to PTV1 is 66 to 74 Gy in 2-Gy fractions or up to 81.6 Gy in 1.2-Gy fractions. One notable exception is early-stage laryngeal cancer, because these tumors are generally treated with 63 to 65.25 Gy in 2.25-Gy fractions. The elective nodes are contoured in a separate treatment volume and are generally treated to 44 to 64 Gy.
For patients receiving adjuvant RT, preoperative imaging should be used to determine the postoperative bed based on the location of the initial tumor. A CTV should be constructed to include the postoperative bed and the pathologically involved neck. An expansion should then be performed to create a PTV. The dose to this region is 63 to 66 Gy for close or positive margins and 60 Gy for negative margins.
The typical recommendations for nodal coverage are based on the T-stage, N-stage, and the primary site. For most midline tumors, bilateral elective nodal coverage is recommended. A few institutions have shown the feasibility of treating well-lateralized tonsillar tumors with ipsilateral radiation alone. Reports from the University of Florida and Princess Margaret Hospital have shown good local control rates in patients treated with ipsilateral radiation for tonsillar carcinoma, with contralateral failure rates of approximately 3% to 4%.
Dose Constraints
The total dose of radiation therapy is limited by the surrounding normal structures. Table 2 shows some of the common dose-limiting structures and the dose constraints that treating physicians try to achieve.
| Structure | Toxicity | Dose Constraint Goal |
|---|---|---|
| Oral cavity | Mucositis | Maximum <55 Gy or 1% cannot exceed 65 Gy |
| Parotid | Xerostomia | Mean <26 Gy |
| Larynx | Aspiration | Mean <45–48 Gy |
| Inferior pharyngeal constrictor muscles | Stricture/aspiration | Mean <54 Gy |
| Mandible | Osteoradionecrosis | Maximum <70 Gy |
| Brachial plexus | Brachial plexopathy | Maximum ≤60 Gy |
| Spinal cord | Transverse Myelitis | Maximum <45 Gy |
Radiation Therapy Techniques
Treatment planning for HNC is based on CT imaging at most institutions in the United States. When using CT-based planning, the target volumes (primary site/postoperative bed and elective nodes) are delineated on the CT scan at the time of simulation and then a plan is devised to deliver radiation to these target volumes via multiple beams. This type of radiation delivery is referred to as three-dimensional conformal radiation therapy (3D CRT), which delivers radiation therapy to the target and minimizes the dose to the surrounding tissues. Before the widespread use of 3D CRT, two-dimensional (2D) radiation was planned, which involved treatment based on anatomic landmarks identified on radiograph imaging.
In an attempt to decrease radiation-induced toxicities in patients with HNC, several alternative RT modalities have been developed. Many institutions are using a newer method of radiation delivery, called intensity-modulated radiation therapy (IMRT). IMRT is similar to 3D CRT, but the intensity of radiation can be varied across each beam. IMRT uses smaller safety margins and higher dose gradients, allowing for further sparing of the surrounding normal structures and thus decreasing toxicity. Two randomized studies have compared salivary flow and xerostomia in patients with early-stage nasopharyngeal treated with IMRT versus 2D radiation therapy. Both of these studies found that the salivary flow rates were improved with IMRT. The study by Kam and colleagues showed that there was improvement in observer-rated severe xerostomia at 1 year, but there was no difference in patient-related outcomes. A third study by Nutting and colleagues compared IMRT with 2D radiation in early and locally advanced pharyngeal tumors. The investigators showed an improvement in xerostomia (greater than grade 2) at 18 months in the IMRT group compared with the conventionally treated group. These studies suggest a benefit to using IMRT.
Because small changes in the patient’s position can have a large impact on the estimated RT field when using IMRT, verifying the patient’s position before initiation of therapy is important. For this reason, image-guided RT (IGRT) is essential during treatment. Generally with IGRT, images are taken in the treatment position before the radiation is delivered. Some of the common forms of IGRT in HNC include MV radiographs, kV radiographs, or cone-beam CT (CBCT) scans. kV images are used to assess the bony anatomy and compare the digitally reconstructed images from the initial planning simulation scan with the image in the treatment position. CBCT is a form of 3D imaging, which involves taking a CT scan using the treatment machine on the day of treatment. CBCT scans can better delineate the soft tissues in the head and neck region and can help account for rotational shifts, which is not possible with radiographic imaging. Another benefit of using IGRT is that if large shifts are required on a daily basis, the physician can choose to resimulate or replan the patient.
There is interest in using proton therapy for the treatment of HNC. The benefit of proton therapy is that the energy from the beam is deposited at a particular depth (the Bragg peak) and there is rapid dose falloff beyond this point. Therefore, proton therapy can be useful to treat tumors close to critical structures and decrease the integral dose to the patient. Until recently, the disadvantage of using proton therapy for head and neck tumors was that the most common technique (passive scatter) used to deliver the treatment did not deliver the radiation as conformally as in other treatment techniques, such as IMRT. However, intensity-modulated proton therapy (IMPT) has recently been used in certain centers. IMPT takes advantage of principles of both IMRT and proton therapy, in that it is able to conform to the tumor volume and minimize low dose to the surrounding structures. Clinical outcomes from IMPT are awaited, but dosimetric studies have shown that the use of IMPT can decrease dose to surrounding critical structures, such as brainstem, spinal cord, the larynx, and parotids.
Principles of chemotherapy
Postoperative CRT
Postoperative CRT has been shown to decrease locoregional recurrence rates in patients with high-risk pathologic features. Two phase III trials, the RTOG 9501 and the EORTC 22931, have investigated the role of postoperative concurrent CRT in this setting. Both trials enrolled patients with high-risk pathologic features defined as the presence of positive margins, perineural invasion, lymphovascular invasion, extracapsular extension outside the involved lymph nodes and multiple positive lymph nodes.
In the RTOG 9501 trial, 459 patients were randomized to receive cisplatin 100 mg/m 2 on day 1 of each cycle every 3 weeks for a total of 3 cycles with concurrent conventional RT (66 Gy over 6.5 weeks) versus conventional RT alone. There was a significant increase in disease-free survival and a decrease in locoregional recurrence, which was the primary end point, with 2-year locoregional control rates of 82% in the concurrent cisplatin/RT group versus 72% in the RT group. There was no benefit in OS, and the incidence of acute severe adverse events was significantly higher in the combined group (77%) compared with the RT group (34%).
In the EORTC 22931 trial, 334 patients were randomized to receive conventional RT alone (66 Gy over 6.5 weeks) versus concurrent RT with cisplatin 100 mg/m 2 every 3 weeks for 3 cycles. The primary end point was 5-year progression-free survival (PFS), which was 47% in the cisplatin/RT group versus 36% in the RT group. There was also a significant decrease in locoregional recurrence rate, with 5-year recurrence at 18% versus 31%, respectively, and a significant increase in the 5-year OS at 53% versus 40%. Severe toxicities (mucositis, myelosuppression) were more frequent (41%) in the concurrent cisplatin/RT group compared with the RT group (21%), but the incidence of late adverse events was the same.
Both trials showed that adding cisplatin does not decrease the rate of distant recurrence, which was 25% with RT versus 21% with concurrent CRT in the EORTC 22931 trial. A subsequent pooled analysis 1 year later by Bernier and colleagues showed that the pathologic factors most associated with survival benefit from postoperative concurrent CRT were positive margins and extracapsular extension.
Definitive or Concurrent CRT
This approach has particular significance in locally advanced oropharyngeal, hypopharyngeal, and laryngeal cancer, in which organ preservation is of paramount importance to maintain function and quality of life. The first 2 studies that addressed the feasibility of an organ-preservation approach in HNC were the Veterans Affairs Laryngeal Cancer Study Group (VA Larynx) and the EORTC 24891 studies, which investigated an induction strategy in patients with resectable laryngeal, hypopharyngeal, and epipharyngeal cancer ( Table 3 ).
| Clinical Trial | Chemotherapy/Radiotherapy Regimen | N | LCR | Larynx Preservation | OS | DFR | Acute Severe Toxicities |
|---|---|---|---|---|---|---|---|
| Postoperative Concurrent Chemoradiotherapy | |||||||
| RTOG 9501 (2004) LAHN | Cisplatin + RT vs RT (RT: standard) | 459 | 2 y 82% 72% ( P = .01) | NA | NS | ≥Grade 3 77% 34% ( P <.001) | |
| EORTC 22931 (2004) LAHN | Cisplatin + RT vs RT (RT: standard) | 334 | 5 y 18% 31% ( P = .007) | NA | 5 y 53% 40% ( P = .02) | ≥Grade 3 41% 21% ( P = .001) | |
| Concurrent Chemoradiotherapy | |||||||
| RTOG 9111 (2003) LA laryngeal cancer | Cisplatin + RT vs induction cisplatin/5FU + RT vs RT (RT: standard) | 547 | 2 y 78% 61% 56% ( P <.05) | 2 y 88% 75% 70% (cis RT c/t induction P = .005, cis/RT c/t RT P <.001, induction c/t RT NS) | 2 y 74% 76% 75% (NS) | 5 y 12% 15% 22% (cis RT c/t RT, P = .03) | ≥Grade 3 toxicities (acute and chronic) 82% 81% 61% (cis RT c/t both other modalities p <.05) |
| Bonner et al (2006) LAHN | Cetuximab + RT vs RT (RT: standard, AF or HF) | 424 | 3 y 47% 34% ( P <.01) | NA | 3 y 55% 45% ( P = .05) | 2 y 16% 17% (NS) | ≥Grade 3 toxicities: NS except for rash with cetux |
| Agarwala et al (2007) | Carboplatin/paclitaxel + RT (RT: standard, boost AF) | 55 | Not reported | NA | 5 y 35% | NA | ≥Grade 3 toxicities: 30% |
| Sequential CRT | |||||||
| VA Larynx Study (1991) | Induction cisplatin/5FU + RT vs surgery + RT | 332 | LCR recurrence: 12% 2% ( P = .0005) | 5 y 66% | 2 y 68% 68% ( P = .09) | Grade 2 mucositis 38% 24% ( p -value not reported) | |
| EORTC 24891 (1996) Laryngeal ca | Induction cisplatin/5FU + RT vs surgery + RT | 202 | LCR recurrence: 33% 30% ( P not available) | 10 y >50% | 10 y 13.1% 13.8% (NS) | 28% 36% ( P not available) | Limiting toxicities: 14% |
| TAX 323 (2007) Unresectable HNC | Induction docetaxel/cisplatin/ 5FU + RT vs induction cisplatin/5FU + RT (RT: standard, AF, or HF) | 358 | 85% 81% ( p -value not reported) | NA | 3 y 37% 26% ( P = .02) | 12.9% 10% ( P = .003) | Neutropenia: TPF: 76% vs PF: 52% ( P <.05) Stomatitis: TPF: 4.6% vs PF: 11.2% ( P <.05) |
| TAX 324 (2007) Stage III, IV | Induction docetaxel/cisplatin/5FU + concurrent CRT with carboplatin vs induction cisplatin/5FU + CRT (RT: standard) | 501 | 30% 38% ( P = .04) | NA | 3 y 62% 48% ( P = .006) | 5% 9% (NS) | Neutropenia: 83% vs 56% ( P <.001) Mucositis: 37% vs 38% (NS) |
| DeCIDE (2012) Stage III, IV | Induction docetaxel/cisplatin/5FU + concurrent CRT with docetaxel/hydroxyurea/5FU vs CRT (same regimen) (RT: HF) | 280 | 3 y LCR failure Rate 9% 12% ( P = .5) | NA | 3 y 75% 73% ( P = .7) | 10% 19% ( P = .02) | Neutropenia: 17% Dermatitis: 19% Mucositis: 45% (all arms) |
| Salama et al (2008) Stage III, IV | Induction carbo/taxol + CRT with paclitaxell/hydroxyurea/5FU (TFHX) (RT: HF: cohorts A, B, C/C with lower RT dose, A with higher RT dose) | 222 | 5 y LCR 91% (similar for all groups) | NA | 3 y 68% (similar for all groups) | 5 y DC 87% (similar for all groups) a | Neutropenia:41% Mucositis: 33% (all arms) |
| Kies et al (2010) Stage III, IV | Induction carbo/taxol/cetuximab + concurrent CRT with platinum or surgery or RT (RT: IMRT or standard RT) | 47 | 3 y 2/47: local recurrence | NA | 3 y 91% | 3 y 4/47: distant recurrence | Neutropenia: 21% Dermatitis: 45% |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree