This article reviews the current management of esophageal cancer, including staging and treatment options, as well as providing support for using multidisciplinary teams to better manage esophageal cancer patients.
Key points
- •
Accurate clinical staging using fused positron emission tomography (PET)-CT scans and endoscopic ultrasound (EUS) is imperative to formulate an optimal treatment plan for patients with esophageal cancer.
- •
Surgery provides optimal treatment of suitable patients with stage I or IIA cancer.
- •
Multimodality therapy is the cornerstone of treatment of advanced-stage esophageal cancer.
- •
Palliative therapies for esophageal are varied and depend on patients’ performance status.
Esophageal cancer was diagnosed in approximately 17,460 patients in 2012 and 15,070 succumbed to their disease in the United States. Esophageal cancer is a difficult disease to cure and generally requires the input of multiple disciplines to optimize overall results. Gastroenterologists, medical oncologists, radiation oncologists, and thoracic surgical oncologists as well as nutritionists, clinical psychologists, and nurse navigators all play a part in the decision making and execution of treatment plans. Additional roles for physical therapy and speech pathology are needed at various times.
The optimum treatment depends on patient comorbidities and clinical stage at presentation. Many patients have lost significant weight and are malnourished at presentation. Risk stratification for esophageal cancer has changed over the past 30 years as adenocarcinoma has increased in incidence. Esophageal adenocarcinomas risk factors include gastroesophageal reflux disease, Barrett esophagus, and tobacco abuse. Reflux disease is exacerbated by obesity, dietary habits, and medications.
Multidisciplinary tumor boards and teams have been shown to improve outcomes in treatment of esophageal cancer. By meeting and discussing each patient in a prospective fashion, a consensus on a treatment plan can be made and implemented. The improved outcomes in treatment, especially in surgically resected patients, are related to an inherent selection bias of the group decision. This bias is based on better preclinical staging by appropriate imaging studies and improved patient risk stratification obtained using clinical practice guidelines. Stephens and colleagues found a reduction in perioperative morbidity and mortality and improved survival in a patient cohort using a multidisciplinary team approach. Low and colleagues similarly reported outstanding morbidity and mortality results in resected patients. They credited clinical pathways and tumor board evaluation for improved patient selection leading to better outcomes. This article reviews the multidisciplinary management of esophageal cancer.
Diagnosis
Gastroenterologists typically are the gatekeepers of the esophageal cancer patients. Upper endoscopy is indicated for symptoms as well as evaluation of abnormal radiographs. It is also used in surveillance and biopsy in Barrett esophagus. Endoscopists define the location of the tumor, screen for skip lesions, and obtain the tissue for diagnosis.
The clinical staging of esophageal cancer entails defining the extent of disease. Tumor depth of invasion and length, nodal status, and distant disease are defined by imaging studies and endoscopy. CT and PET scans are the mainstay of excluding distant disease. They provide anatomic location and can visualize potential nodal disease and define distant metastasis. EUS provides more accurate definition of the depth of invasion and nodal status; however, malignant strictures may not be passable in 10% to 38% of the cases necessitating dilation to complete. Neck and abdominal ultrasound and selected MRI can be used as adjuncts to confirm or exclude metastatic disease of the liver, adrenal gland, and cervical nodal disease.
Diagnosis
Gastroenterologists typically are the gatekeepers of the esophageal cancer patients. Upper endoscopy is indicated for symptoms as well as evaluation of abnormal radiographs. It is also used in surveillance and biopsy in Barrett esophagus. Endoscopists define the location of the tumor, screen for skip lesions, and obtain the tissue for diagnosis.
The clinical staging of esophageal cancer entails defining the extent of disease. Tumor depth of invasion and length, nodal status, and distant disease are defined by imaging studies and endoscopy. CT and PET scans are the mainstay of excluding distant disease. They provide anatomic location and can visualize potential nodal disease and define distant metastasis. EUS provides more accurate definition of the depth of invasion and nodal status; however, malignant strictures may not be passable in 10% to 38% of the cases necessitating dilation to complete. Neck and abdominal ultrasound and selected MRI can be used as adjuncts to confirm or exclude metastatic disease of the liver, adrenal gland, and cervical nodal disease.
Staging
The 7th edition of the American Joint Committee on Cancer (AJCC)/International Union Against Cancer Cancer Staging Manual has defined new stage categories for adenocarcinoma and squamous cell carcinoma of the esophagus, driven by surgical resected patient data. It is more complex than the TNM system of the past. Now nonanatomic factors play a role, including histopathologic cell type and grade (G) as well as location. Table 1 provides new definitions and Tables 2 and 3 present the stage breakdown for adenocarcinoma and squamous cell carcinoma. A pictorial matrix is shown in Figs. 1 and 2 . In adenocarcinoma, the grade affects stages IA, IB, and IIA, whereas, grade and location affect stage distribution in squamous cell carcinoma. Several groups have already begun publishing supporting case series evaluating the new stage system and they seem to support the new nodal stratification but not necessarily the grade stratification; however, the data that drove the new system were based on surgically resected disease without neoadjuvant or adjuvant therapy. Future directions may look to the past, when tumor length was part of the staging system. Yendamuri and colleagues and Gaur and colleagues have demonstrated the prognostic significance of postresection tumor length and endoscopic tumor length as predictors of survival, respectively, which may play a role in future staging.
| Tis | Carcinoma in situ/high-grade dysplasia |
| T1 | |
| T1a | Lamina propria or muscularis mucosae |
| T1b | Submucosa |
| T2 | Muscularis propria |
| T3 | Adventitia |
| T4 | |
| T4a | Pleura, pericardium, diaphragm, or adjacent peritoneum |
| T4b | Other adjacent structures: aorta, vertebral body, trachea |
| N0 | No regional nodes |
| N1 | 1–2 Node metastases |
| N2 | 3–6 Node metastases |
| N3 | >6 Node metastases |
| M0 | No distant disease |
| M1 | Distant metastasis |
| Stage | T | N | M | G |
|---|---|---|---|---|
| 0 | Tis | 0 | 0 | 1 |
| IA | 1 | 0 | 0 | 1–2 |
| IB | 1 | 0 | 0 | 3 |
| 2 | 0 | 0 | 1–2 | |
| IIA | 2 | 0 | 0 | 3 |
| IIB | 3 | 0 | 0 | Any |
| 1–2 | 1 | 0 | Any | |
| IIIA | 1–2 | 2 | 0 | Any |
| 3 | 1 | 0 | Any | |
| 4a | 0 | 0 | Any | |
| IIIB | 3 | 2 | 0 | Any |
| IIIC | 4a | 1–2 | 0 | Any |
| 4b | Any | 0 | Any | |
| Any | 3 | 0 | Any | |
| IV | Any | Any | 1 | Any |
| Stage | T | N | M | G | Location |
|---|---|---|---|---|---|
| 0 | Tis | 0 | 0 | 1 | Any |
| IA | 1 | 0 | 0 | 1 | Any |
| IB | 1 | 0 | 0 | 2–3 | Any |
| 2–3 | 0 | 0 | 1 | Lower | |
| IIA | 2–3 | 0 | 0 | 1 | Upper Middle |
| 2–3 | 0 | 0 | 2–3 | Lower | |
| IIB | 2–3 | 0 | 0 | 2–3 | Upper Middle |
| 1–2 | 1 | 0 | Any | Any | |
| IIIA | 1–2 | 2 | 0 | Any | Any |
| 3 | 1 | 0 | Any | Any | |
| 4a | 0 | 0 | Any | Any | |
| IIIB | 3 | 2 | 0 | Any | Any |
| IIIC | 4a | 1–2 | 0 | Any | Any |
| 4b | Any | 0 | Any | Any | |
| Any | 3 | 0 | Any | Any | |
| IV | Any | Any | 1 | Any | Any |
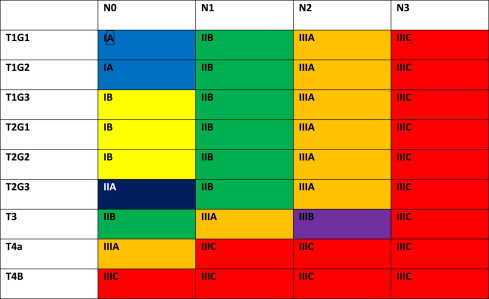
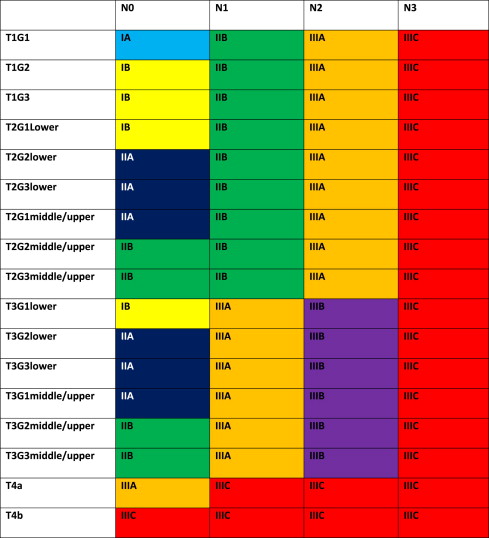
The significance of the new staging system is the impact on pretreatment clinical staging. It is imperative to try and delineate the nodal status based on the number of clinically suspicious nodes. Fused PET-CT scan and EUS will be the mechanisms by which these nodes are quantitated. CT node criteria of mediastinal nodes of 1 cm or greater size in short axis and paraesophageal, perigastric, and celiac nodes greater than 0.7 cm are concerning for metastatic disease, especially when coupled with increased standard uptake value (SUV) greater than 3 and number of PET abnormalities noted. EUS can define pathologic nodes based on sonographic (greater than 1 cm, round, and hypoechoic) characteristics but can be difficult to discern the total number of suspicious nodes.
Assessment
All esophageal cancer patients should be discussed in a multidisciplinary tumor board in a prospective fashion. Ideally, they should be seen and examined by all appropriate specialties. In so doing, a tailored treatment plan for each patient can be developed based on stage, comorbid conditions, and patient desires. Blazeby and colleagues demonstrated a 15% discordant treatment from their original multidisciplinary team plan because of lack of this patient information. To aide in this endeavor, a specialized dedicated esophageal nurse navigator can help the patient and family navigate this evaluation more easily. All appropriate staging studies can be obtained as needed for their plan of treatment and the treatment expedited.
A thorough history and physical examination assess patient performance status and nutritional status. Minimal laboratory evaluation should include complete blood cell count and complete metabolic panel to evaluate nutritional status. Subjective global assessment uses focused history evaluating weight change, dietary intake, gastrointestinal symptoms, and associated comorbidities having an impact on metabolic demand and physical findings, including loss of subcutaneous fat, muscle wasting, edema, and ascites, to subjectively help evaluate nutrition status. Other concerning findings are weight loss greater than or equal to 10%, body mass index less than 20 kg/m 2 , prognostic nutrition index greater than or equal to 50, severe dysphagia, albumin less than 3.5 g/dL, and prealbumin less than 10 mg/dL. A formal nutrition consult is advised for all patients to help maintain their nutrition during their treatment and thereafter by providing patients a better understanding of calorie intake required to maintain adequate nutrition.
Comorbidities should be closely evaluated, especially underlying cardiovascular disease and respiratory issues. Pulmonary function test should be ordered on patients considered for surgery and cardiac stress test as indicated by history and physical examination. Patients should stop tobacco products and alcohol products. A functional assessment should be performed and either Karnofsky score or Eastern Cooperative Oncology Group (ECOG) performance status recorded ( Table 4 ). Risk stratification models have been championed by different groups. Edinburgh Clinical Risk Score evaluates patients based on clinical stage, performance status, rate of weight loss, and C-reactive protein level to assess risk of dying from gastroesophageal cancer. Modified Glasgow prognostic score is another mechanism used to prognosticate, using a combination of C-reactive protein and albumin levels. Crumley and colleagues demonstrated, in a retrospective case series review, that the modified Glasgow prognostic score was a significant predictor of cancer specific survival.
| Definition | Karnofsky Score | ECOG Performance Status | ||
|---|---|---|---|---|
| Normal activity and work, no special need | 100 | No complaint | 0 | Asymptomatic (fully active, able to carry on all predisease activities without restriction) |
| 90 | Minor signs and symptoms disease | 1 | Symptomatic but completely ambulatory (restricted in physically strenuous activity but ambulatory and able to carry out work of light or sedentary nature) | |
| 80 | Normal activity with effort some signs and symptoms of disease | |||
| Unable to work; able to live at home and care for most personal needs; varying amount of assistance needed | 70 | Cares for self; unable to carry on normal activity or to do active work | 2 | Symptomatic, <50% in bed during the day (ambulatory and capable of all self-care but unable to carry out any work activities, up and about more than 50% of waking hours) |
| 60 | Requires occasional assistance but is able to care for most of his personal needs | |||
| 50 | Requires considerable assistance and frequent medical care | |||
| Unable to care for self; requires equivalent of institutional or hospice care; disease may be progressing rapidly | 40 | Disabled; requires special care and assistance | 3 | Symptomatic, >50% in bed, but not bedbound (capable of only limited self-care, confined to bed or chair 50% or more of waking hours) |
| 30 | Severely disabled; hospital admission is indicated although death not imminent. | 4 | Bedbound (completely disabled, cannot carry on any self-care, totally confined to bed or chair) | |
| 20 | Very sick; hospital admission necessary; active supportive treatment necessary | |||
| 10 | Moribund; fatal processes progressing rapidly | |||
| 0 | Dead | 5 | Death | |
Endoscopic therapy
Barrett esophagus is a premalignant mucosal abnormality in the esophagus related to acid or, more likely, bile reflux damage, resulting in dysplastic changes at the squamocolumnar junction. Despite proton pump inhibitors and even fundoplication, Barrett esophagus can be resilient. New endoscopic therapies are available to treat dysplastic changes in the esophageal mucosa. The risk of cancer is increased and may be as low as 0.6% per year in low-grade dysplasia and up to 5% per year in high-grade dysplasia. A routine surveillance program is recommended for Barrett esophagus with surveillance endoscopy with biopsies using Seattle protocol or other biopsy protocols on a regular basis.
Argon plasma coagulation (APC) was one of the first therapies for mucosal ablations. It is used in both treatment of premalignant disease and palliation of advanced cancer. Complete eradication of dysplasia and early cancers is seen in 68% of the patients. Complications occur in approximately 24% of the procedures, most of which are self-limited, such as pain, bleeding, ulceration, and stricture. There is also a finite risk of developing buried glands; exact risk is uncertain.
Photodynamic therapy has demonstrated efficacy in eradicating both premalignant and early-stage tumors. Used in combination with proton pump inhibitors, a 77% complete response is noted in high-grade dysplasia and stage I carcinomas. The main drawbacks are the photosensitivity and an increased stricture rate. Buried glands have been noted in 24% to 44% of the patients and recurrent cancers have been seen in 10%.
Radiofrequency ablation (RFA) is an effective treatment of Barrett esophagus. Shaheen and colleagues, in a randomized trial, demonstrated 81% complete response in high-grade dysplasia and there was less progression of disease and fewer cancers noted in follow-up. The main side effect was chest pain and the stricture rate of 6%. RFA is good for flat Barrett mucosa but not as effective for lumpy-bumpy (nodular) disease because of loss of probe contact.
Cryotherapy uses low-pressure liquid nitrogen spray delivered during endoscopy with a vented stomach. Greenwald and colleagues, in a multi-institutional study, demonstrated the efficacy of cryotherapy in high-grade dysplasia and intramucosal carcinoma. Dumot and coworkers demonstrated a 90% response rate and their 2-year results showed 68% downgrade of high-grade dysplasia and an 80% complete response in intramucosal cancer. Multiple procedures are required for complete eradication; and side effects included mild pain and minor strictures. It is better suited for lumpy-bumpy Barrett esophagus.
Unlike the previously described endoscopic procedures, endoscopic mucosal resection (EMR) provides a pathologic specimen for review. Several techniques have been used, including submucosal injection with snare polypectomy, cap-assisted mucosal resection, and band ligator-assisted mucosal resection. In case series, Barrett esophagus was completely eradicated in 75% to 96% of the patients and dysplasia or cancer complete response in 85% to 100%. Complications include bleeding and stricture. Ideal lesions are less than 2 cm in diameter, involve less than one-third of the circumference, and are confined to the mucosa.
Surgery
Surgery has been the cornerstone of curative treatment of esophageal cancer for the past several decades. The overall surgical outcomes have been improving, with most recent data demonstrating surgical mortality rates between 0.3% and 7.2% and rates of morbidity between 30% and 68% ( Table 5 ). Population-based studies, however, reflect more disparate outcomes compared to these case series. Kohn and colleagues, using the Nationwide Inpatient Sample in 2007, found the esophagectomy mortality rate was 7% and pointed to improved outcomes in higher-volume centers. Ra and colleagues at the University of Pennsylvania, during approximately the same time period, using Survival, Epidemiology and End Results Program data, found a mortality rate of 14%, and risk factors included advancing age, comorbidities, and hospital volume. The bottom line is that proper patient selection leads to better outcomes and, hence, the disparity of results seen in case series where selection bias may occur.
| Study | N | Sex (M/F) | Stage I | Stage II | Stage III | Stage IV | Median Survival (Mo) | 5-Y (%) | % Neoadjuvant RC | % Hospital Morbidity | % Hospital Mortality |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Atkins et al, 2004 | 379% | 307/72 | NR 22.1% | NR 51.7% | NR 18.8% | NR 5.1% | NR | NR | 44.1 | 64 | 5.8 |
| Mariette et al, 2004 | 386% | 347/39 | 51 13.2% | 133 34.5% | 169 43.8% | 0 0 | 28.9 | 31 | 50.2 | 36.3 | 3.6 |
| Portale et al, 2006 | 263% | 215/48 | 97 36.8% | 63 23.9% | 93 35.4% | 10 3.8% | >48 | 46.5 | 18 | 62 | 4.5 |
| Omloo et al, 2007 | 205% | 178/27 | NR 26.9% a | NR | NR 73.1% | 0 | THE—34% TTE—36% | 0 | NR | 3 | |
| Low et al, 2007 | 340% | 241/99 | 87 25.6% | 133 39.1% | 94 27.6% | 9 2.6% | NR | NR | 43 | 45 | 0.3 |
| Adams et al, 2007 | 330% | 224/106 | NR 1.8% | NR 29% | NR 56% | NR 11% | 22 | 26 | 27.6 | 30.7 | 7.2 |
| Sundelof et al, 2008 | 232% | 193/39 | 52 22% | 61 26% | 70 30% | 38 16% | NR | 25 | 23.0 | 33.2 | 3.9 |
| Malin et al, 2009 | 221% | 191/30 | 24 10.8% | 77 34.8% | 66 29.9% | 4 1.8% | NR | 33 | 42.1 | 41.6 | 2.3 |
| Wouters et al, 2009 | 214% b | 159/54 | 31 14.5% | 82 36.3% | 74 34.6% | 15 7% | 22 | NR | 25.2 | 66.8 | 4.7 |
| Hsu et al, 2010 | 392% | 374/18 | 42 10.7% | 151 38.5% | 116 29.6% | 83 21.2% | 20 | 27.1 | 0 | NR | NR |
| Cijs et al, 2010 | 1061% | 795/266 | 17 1.6% | 573 54.0% | 306 28.8% | 116 10.9% | 20.5 | 27 | 38.6 | 39 | <70:5.4 >70:11.6 |
| Pultram et al, 2010 | 234% | 196/38 | 28 12.0% | 87 37.1% | 105 44.9% | 14 6.0% | 26 | 33 | 0 | 61 | 6.2 |
a Omloo’s pathologic combined stage I–II.
Approximately one-third of the patients who present with esophageal cancer are resection candidates. Overall cure rates with surgical resection are between 25% and 35% at 5 years. The new 7th edition AJCC staging classification was based purely on patient outcomes from surgically resected patients—all pathologically proven disease. Five-year survival rates for each of the adenocarcinoma stages are stage IA, 78%; stage IB, 64%; stage IIA, 50%; stage IIB, 39%; stage IIIA, 25%; stage IIIB, 17%; and stage IIIC, 14%.
Ivor Lewis esophagogastrectomy is the most common procedure performed for esophageal cancer. It involves laparotomy with mobilization of the stomach based on the right gastroepiploic artery. A right thoracotomy is then performed and the thoracic esophagus and its surrounding nodes mobilized. The gastric conduit is brought into the chest and then an esophagogastrectomy performed with 8-cm proximal and 5-cm distal margins. An intrathoracic anastomosis is then performed and the chest widely drained. This approach provides the optimum exposure of the esophagus and its nodal basins. It, however, is a big insult on patients already debilitated by the underlying disease. The morbidity of the procedure is higher but overall survival rates seem better from a cancer standpoint.
Transhiatal esophagectomy is the next most common procedure performed. It involves a similar laparotomy but a neck incision is performed in lieu of thoracotomy. The intrathoracic esophagus is bluntly and at times blindly mobilized. The proximal esophagus is divided in the neck and the specimen brought back into the abdomen and the esophagogastrectomy performed. The gastric conduit is then brought up to the neck, generally in the orthotopic position, and cervical anastomosis performed. This approach resects more esophagus but at the expense of oncologic node dissection. The morbidity of the operation in most studies is less and seems better tolerated by marginal surgical candidates. The anastomosis, however, is under more tension and tends to leak more often.
The McKeown 3-incision esophagectomy optimizes the benefits of both the approaches described previously. It is the optimal approach for treating upper thoracic esophageal cancers in the middle to upper third. The modified procedure is started with a right thoracotomy where the esophagus is mobilized en bloc along with a thorough lymph node dissection. The chest is drained widely and closed and the patient repositioned supine. A laparotomy is performed and the gastric conduit mobilized in a similar fashion, with a lymph node dissection. A left lateral neck incision is performed and the cervical esophagus mobilized, the proximal margin determined, and the esophagus transected. As in the transhiatal esophagectomy, the specimen is brought back into the abdomen, the distal margin determined, and gastric conduit fashioned. The conduit is brought up into neck in the orthotopic position and a cervical anastomosis performed. This approach allows for a 3-field lymphadenectomy to be performed along with the benefits of a neck anastomosis.
Left thoracotomy with phrenotomy approach for esophagectomy was first championed by Churchill and Sweet, in 1942, when they published a case series of 11 patients. This approach entails a left thoracotomy for exposure through the 6th or 7th interspace. The diaphragm is then opened in a radial fashion exposing the abdominal contents. The gastric conduit is mobilized along with the distal esophagus. The tumor is resected and an intrathoracic anastomosis completed. The downside of this incision is the limited abdominal exposure and inability to place feeding access. Also, margins of resection may be compromised or at least limited by this procedure.
A left thoracoabdominal esophagogastrectomy with left neck incision is another approach that provides excellent exposure for cardia and gastroesophageal juncture tumors. A left thoracoabdominal incision is made along left chest extending into the abdomen via an oblique incision. The costal arch is divided and diaphragm is opened radially exposing the gastroesophageal juncture. The short gastrics are easily controlled and divided and the stomach mobilized along with the distal esophagus. A radical lymph node dissection is performed. An oblique left neck incision is performed and the cervical esophagus exposed and mobilized with blunt and sharp dissection. The esophagogastrectomy is performed and a cervical anastomosis created.
Minimally invasive esophagectomy (MIE) was first performed in the mid 1990s. It has been slow to gain a foothold with surgeons. It is a challenging procedure that can be performed via different approaches, most are modifications of the standard open procedures (described previously). Laparoscopy with thoracoscopy (Ivor Lewis), thoracoscopy with laparoscopy and left neck incision (McKeown esophagectomy), and laparoscopy with neck incision (transhiatal) are a few of the common procedures. Luketich and coworkers published one of the largest series of MIE, 222 patients. MIE was successfully performed in 92.8% with an operative mortality of 1.4%, demonstrating the safety, feasibility, and equivalent outcomes of open techniques. Butler and colleagues did an extensive review of the current literature on MIE and demonstrated comparable results from the oncologic standpoint and comparable morbidity and mortality to standard open procedures. It has, however, yet to be proved to offer improved outcomes. It is a technique all thoracic surgeons should have in their armamentarium but the learning curve is steep.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




