There is a compelling need for close coordination and integration of multiple specialties in the management of patients with early-stage breast cancer. Optimal patient care and outcomes depend on the sequential and often simultaneous participation and dialogue between specialists in imaging, pathologic and molecular diagnostic and prognostic stratification, and the therapeutic specialties of surgery, radiation oncology, and medical oncology. These are but a few of the various disciplines needed to provide modern, sophisticated management. The essential role for coordinated involvement of the entire health care team in optimal management of patients with early-stage breast cancer is likely to increase further.
Key points
- •
The management of early-stage breast cancer represents one of the most complete examples of the value of multidisciplinary cancer care, and includes a wide range of disciplines beginning with, but not limited to, radiology, pathology, surgery, radiation therapy, and medical oncology.
- •
Optimal evaluation, diagnosis, staging, treatment, and surveillance of patients with breast cancer require integration of a broad range of specialized expertise.
- •
The considerable progress made in clinical outcomes for patients with breast cancer may likely relate in large part to the success of multidisciplinary and integrated delivery of health care to these patients.
Introduction
The management of early-stage breast cancer (ESBC) represents arguably the earliest and most complete example of the value of multidisciplinary cancer care. It goes far beyond the disciplines of diagnostics and interventional radiology, pathology, surgery, radiation oncology, and medical oncology discussed in the paragraphs to come. It also includes other primary care specialties such as family medicine, internal medicine, obstetrics and gynecology, as well as the important participation of nursing, social work, and physical and occupational therapy, among many others. The optimal evaluation, diagnosis, staging, primary treatment, adjunctive therapy, supportive care, and long-term monitoring or surveillance require access to a broad wealth of specialized expertise and the integrated involvement of nearly all participants of modern health care delivery. In fact, it is in no small part the success of multidisciplinary and integrated care of patients with ESBC to which we can attribute the outstanding progress in clinical outcomes for patients with this disease, far outpacing successes in many other adult solid tumors. Within the limitations of this summary, this article focuses attention on the role of the radiologist, pathologist, surgeon, radiation oncologist, and medical oncologist in the management of patients during the initial presentation of breast cancer.
Introduction
The management of early-stage breast cancer (ESBC) represents arguably the earliest and most complete example of the value of multidisciplinary cancer care. It goes far beyond the disciplines of diagnostics and interventional radiology, pathology, surgery, radiation oncology, and medical oncology discussed in the paragraphs to come. It also includes other primary care specialties such as family medicine, internal medicine, obstetrics and gynecology, as well as the important participation of nursing, social work, and physical and occupational therapy, among many others. The optimal evaluation, diagnosis, staging, primary treatment, adjunctive therapy, supportive care, and long-term monitoring or surveillance require access to a broad wealth of specialized expertise and the integrated involvement of nearly all participants of modern health care delivery. In fact, it is in no small part the success of multidisciplinary and integrated care of patients with ESBC to which we can attribute the outstanding progress in clinical outcomes for patients with this disease, far outpacing successes in many other adult solid tumors. Within the limitations of this summary, this article focuses attention on the role of the radiologist, pathologist, surgeon, radiation oncologist, and medical oncologist in the management of patients during the initial presentation of breast cancer.
Breast imaging
Breast imaging plays a central role in the multidisciplinary care of patients with breast cancer. With advances in conventional imaging, the advent of new imaging modalities, and the introduction of percutaneous needle biopsy techniques, the breast imager must be fully integrated into the care team to optimize planning for surgical, medical, and radiation therapy for patients with a new diagnosis of breast cancer. Detection of breast cancer, particularly in its earliest stages, is especially dependent on high-quality imaging. One of the fundamental advances in breast imaging is the development of full-field digital mammography, providing substantially better contrast than conventional analog imaging and providing an excellent platform for additional image evaluation such as computer-aided detection. State-of-the-art ultrasound systems now offer high-resolution imaging with high-frequency transducers and digital processing techniques such as spatial compounding and harmonic imaging. More recent imaging advances include dynamic contrast-enhanced breast magnetic resonance imaging (MRI) with dedicated breast-imaging coils and, most recently, molecular imaging techniques that provide contemporary versions of earlier nuclear medicine techniques.
The Breast Imaging Reporting and Data System, or BI-RADS, includes a lexicon of terms for describing the morphology of breast lesions, including 7 standardized final-assessment categories from 0 to 6, along with a final conclusion of the level of suspicion and any recommendation for additional imaging or biopsy-reducing ambiguity in breast-imaging reports. New image-guided tissue sampling techniques allow histologic evaluation of almost any breast lesion, and large-core needle biopsies are now routinely performed. Close communication between radiologists and pathologists is essential to confirm concordance between imaging and histology and avoid false-negative results.
Optimal surgical planning requires the breast imager to accurately determine the size of the primary lesion, the presence of multifocal or multicentric lesions, and the presence of contralateral disease. Mammography and ultrasound imaging are very useful but MRI may provide more accurate assessment of preoperative staging, although the high rate of false-positive findings remains a concern. In addition to diagnostic confirmation and accurate imaging measurements of cancer size, evaluation of needle biopsy samples provides hormone receptor and HER2 status of cancerous lesions, all of which are essential for the medical oncologist when considering hormonal therapy and whether to administer neoadjuvant rather than adjuvant therapy.
Preoperative MRI is clearly more accurate in the determination of the size of the primary lesion, the presence of multifocal and multicentric disease, the presence of contralateral disease, and the presence of axillary adenopathy. However, MRI has not been shown to reduce rates of reoperation or in-breast recurrence, and no survival benefit has been demonstrated. Conversely, studies have demonstrated that patients undergoing preoperative MRI have higher mastectomy rates than those not undergoing MRI. It is important that the multidisciplinary care team decide whether preoperative MRI is warranted in patients with a new diagnosis of breast cancer.
Pathology
The pathologist, though arguably the least visible member of the multidisciplinary breast team, plays a crucial role in patient management. Pathologic diagnosis represents the critical first step for subsequent evaluation and treatment of patients with breast cancer. However, the role of the pathologist extends far beyond providing accurate and complete information on the biology of a given breast carcinoma (grade, biomarkers) and the extent of disease (tumor size, regional lymph node involvement). Without appropriate clinical and radiologic information, a proper pathologic workup is often not possible and may be inaccurate. The pathologist acts as a consultant to the clinicians, as illustrated by the importance of multidisciplinary patient-care conferences where the pathologist clarifies and elaborates on diagnostic reports. The pathologist also directs and oversees quality assurance of biomarker testing and the selection of optimal tissue blocks for special studies and clinical research protocols that have a correlative science component involving human tissues. At referral institutions, pathologic material from other hospitals is reviewed, providing a second opinion and leading to a change in treatment recommendations in a significant percentage of cases. Fig. 1 illustrates 2 examples of significant diagnostic discrepancies. Fig. 1 A shows a needle-core biopsy that had been diagnosed elsewhere as high-grade ductal carcinoma in situ (DCIS) but revealing a patchy infiltrate of small discohesive atypical cells that had not been appreciated. A cytokeratin immunostain (see Fig. 1 B) demonstrated the epithelial origin of the infiltrate, and a diagnosis of invasive lobular carcinoma was made. Another needle-core biopsy came with a diagnosis of invasive ductal carcinoma (see Fig. 1 C). However, at higher power the small nests of atypical cells clearly were enveloped by myoepithelial cells (see Fig. 1 D) that were further highlighted on immunohistochemical (IHC) stains, and a diagnosis of papillary carcinoma in situ was rendered with entrapment of atypical epithelium within the sclerosing stroma.
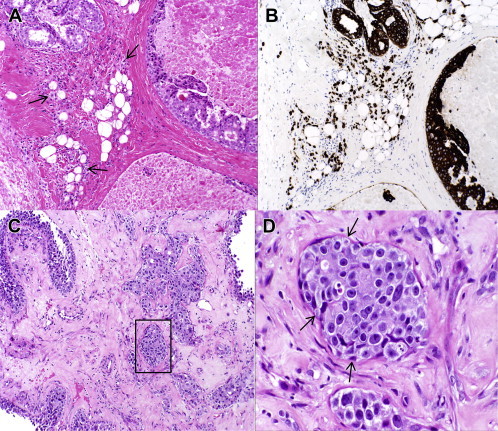
The most important distinction a pathologist has to make and, fortunately, the area of highest interobserver concordance (95.5%–98%) is between invasive mammary carcinoma and noninvasive disease. However, the distinction between invasive carcinoma and noninvasive disease is sometimes difficult even with the help of IHC stains, especially when there is a paucity of diagnostic material. Perhaps the lowest degree of concordance is in the area of atypical intraductal proliferations such as the distinction between DCIS and atypical ductal hyperplasia (ADH). Likewise, precise determination of tumor size may be surprisingly difficult, especially for DCIS lesions, tumors with a multinodular architecture, and carcinomas treated with neoadjuvant chemotherapy. While the importance of wide surgical margins remains in dispute, a change in margin status on pathology review is uncommon (2%–6.5%). Significant interobserver variability has been reported when assessing lymphovascular invasion. The College of American Pathologists and the American Society of Clinical Oncology (ASCO) issued guidelines for HER2 and ER/PR testing in 2007 and 2010 in an effort to reduce the well-documented interlaboratory variability in ER/PR/HER2 testing. Although breast tumor grade is a powerful prognostic factor, its clinical utility is diminished by the well-described interobserver variability. Although centrally performed multigene tests such as the Oncotype DX have good technical reproducibility, they may be subject to significant intratumor variability. Moreover, much of the information provided by commercial multigene assays may be redundant and may overlap with routine data elements in pathology reports. The complexity and often subjective nature of the reported results makes review of the pathologic material by an expert breast pathologist an essential part of multidisciplinary management of breast cancer.
Surgery
The mainstay of treatment for ESBC including invasive disease and DCIS continues to be surgery, in the form of either breast-conserving therapy (BCT, lumpectomy) or mastectomy following or before adjuvant therapy. Clearly the trajectory of the surgical management of ESBC has been toward less aggressive and debilitating surgical procedures. Current guidelines from the National Comprehensive Cancer Network (NCCN) offer 2 options for the surgical treatment of the breast for ESBC: (1) BCT with or without adjuvant radiation or (2) mastectomy. Important determinants of breast treatment are patient preference, the size and/or multifocality of tumor, competing comorbidities, and patient age.
The area of greatest controversy in breast surgery is the management of the axillary nodes. One of the most important prognostic factors with respect to survival from ESBC is the presence of axillary lymph node involvement. Therefore, determining the status of the axillary lymph nodes is critically important and is used to guide further therapy. Until the 1970s, essentially all breast cancers were treated with a radical mastectomy, including excision of level-I, -II, and -III nodes as well as the pectoralis major muscle and overlying skin. With respect to metastases to axillary nodes, this technique was both diagnostic and therapeutic but was associated with significant morbidity. Once studies demonstrated that modified radical mastectomy was equally effective in prolonging survival, this procedure replaced radical mastectomy with axillary dissection largely limited to excision of level I and level II lymph nodes. Similarly, once the combination of lumpectomy and axillary dissection with postlumpectomy radiation therapy was found to be equivalent to modified radical mastectomy with respect to survival from breast cancer, all patients continued to undergo standard axillary dissection of level-I and -II nodes. Subsequent large retrospective studies suggested a small survival advantage for patients with breast cancer undergoing axillary node dissection, whereas other studies failed to demonstrate any such advantage.
At the same time, lymphatic mapping with sentinel node biopsy (SNB) was developed and was quickly adopted by surgeons throughout the Unites States. Although the combined use of radiolabeled sulfur-colloid and isosulfan blue dye increases the success of identifying the sentinel nodes, the routine use of SNB has been associated with a false-negative rate of approximately 2% to 10%, which should be discussed with the patient. In situations where the surgeon is unable to identify the sentinel nodes, axillary node dissection is generally recommended. SNB is now the standard of care for most patients with clinically node-negative ESBC. For those patients with negative sentinel nodes, no further lymph node dissection is necessary. In patients with a positive SNB node undergoing completion lymph node dissection (CLND), additional nodal involvement has been found in approximately 20% to 50% of patients. Although a CLND continues to represent the standard of care in patients with a positive SNB, it may not confer benefit in all patients based on the results of ACOSOG Z0011, which showed equivalent disease-free survival and overall survival in patients with clinical T1-T2 disease with 1 to 2 positive sentinel nodes randomized to CLND and radiation or to radiation alone. For those patients with positive sentinel nodes who do not undergo CLND, many radiation oncologists include the axillary nodes in the radiation field, which may reduce axillary recurrence. At present, it is recommended that women undergoing mastectomy with a positive SNB undergo excision of level-I and -II axillary nodes.
The pathologic evaluation of the nodes has also undergone notable changes in recent years. Based on several studies, and as defined in the most recent American Joint Cancer Committee staging manual, a SNB with micrometastases (<0.2 mm diameter) is now considered negative. Likewise, breast-cancer cells detected only by IHC staining, but not visualized by hematoxylin and eosin staining, are also now considered negative, with many institutions no longer performing IHC analysis of sentinel nodes. Additional clinical trials should help clearly determine which patients with breast cancer and with positive SNB can avoid further axillary dissection. SNB is generally not recommended for DCIS and is of uncertain benefit even in patients with microinvasive breast cancer. On the other hand, in patients with clinically involved axillary nodes, metastatic involvement should be confirmed by fine-needle aspiration (FNA) or core-needle biopsy, and a standard axillary dissection should be performed with no role for SNB.
Locally advanced disease includes breast cancers that present with large primary tumors (>5 cm), skin or chest-wall involvement, or advanced lymph node disease. The initial approach to locally advanced breast cancer is similar to ESBC, with special attention to physical examination and imaging to evaluate the extent of local involvement including skin involvement, fixation to the chest wall, and fixed or matted nodes that will dictate locoregional management. Additional imaging to rule out distant metastases is important, as these patients are at increased risk for metastatic disease. A multidisciplinary team approach is particularly important for these complex patients who require multimodality therapy. Neoadjuvant chemotherapy has become the standard approach for patients with nonoperable breast cancer to convert them to operative candidates and facilitate local control. For patients with operable breast cancer, neoadjuvant chemotherapy has been shown to improve rates of resectability if undergoing BCT, with disease-free and overall survival similar to that of adjuvant chemotherapy. Recently, neoadjuvant endocrine therapy with aromatase inhibitors has been shown to provide comparable response rates and breast-conservation rates in selected patients. Following chemotherapy, either mastectomy or BCT is performed based on tumor response and patient choice. Contraindications to breast conservation include multicentric tumors, extensive calcifications, extensive skin involvement, and inability to obtain negative margins. Radiation therapy is standard after BCT, whereas postmastectomy radiation is based on disease stage at presentation. Inflammatory breast cancer (IBC) must be distinguished from both locally advanced breast cancer, which arises more slowly with infrequent or focal skin involvement, and mastitis, which may delay treatment. Although the diagnosis of IBC is based on clinical findings, dermal lymphatic involvement caused by tumor emboli within the dermal lymphatics is specific for inflammatory breast cancer but is seen in only 75% of cases. Clinically suspicious lymph nodes should be biopsied to confirm nodal involvement and accurately stage the patient. SNB is not indicated for IBC because of a high false-negative rate likely attributable to involvement and consequent occlusion of the dermal lymphatics by tumor emboli. Additional imaging to evaluate involvement of distant sites should be performed, as more than 30% of patients may have metastatic disease. Neoadjuvant chemotherapy followed by modified radical mastectomy then by postmastectomy chest-wall radiation should be performed for patients who respond to chemotherapy and in whom complete resection can be performed. Patients with inoperable tumors despite chemotherapy should be treated with preoperative radiation followed by mastectomy if feasible.
Although surgery remains an important mainstay of treatment for locally advanced breast cancer, the more widespread use of effective and targeted systemic therapy before surgery has allowed for more successful treatment of advanced disease as well as improved understanding of tumor biology in vivo. It is hoped that systemic therapy will continue to improve disease-specific mortality in these high-risk patients, allowing for further reduction of surgical extent without compromise of patient outcomes.
Radiation oncology
Radiation therapy has been used, in conjunction with surgery, as a critical component of BCT since the 1970s. The initial goal of radiation in this setting was to minimize the risk of local recurrence and, therefore, the need for either immediate or delayed mastectomy. Over time, as data have matured and long-term follow-up has accumulated, it has become clear that a significant reduction in the risk of local recurrence also improves survival. As a result, postlumpectomy radiotherapy is indicated for almost all patient subgroups to reduce the risk of local regional recurrence by approximately two-thirds and improve overall survival over lumpectomy alone.
Historically, postlumpectomy radiotherapy has been delivered over 4.5 to 5 weeks to the whole breast followed by a 1 to 1.5-week “boost” focused on the lumpectomy cavity. However, recent international clinical trials have demonstrated that an accelerated whole-breast treatment delivering larger doses per treatment over a shorter period of time and to an overall lower total dose is comparable with standard treatment in both efficacy and toxicity. Although the overall total dose is lower, the treatment is believed to be “biologically equivalent,” owing to the more profound impact of larger daily treatments. In the Canadian study that recently reported outcomes at 10 years, recurrence rates in both the standard and accelerated arms were just over 6%, with approximately 70% of women in both arms experiencing good or excellent cosmetic results. This regimen has become a widely accepted alternative to standard therapy in the appropriate patient.
Another evolving technique designed to limit normal tissue toxicity and patient inconvenience is partial breast irradiation (PBI). PBI focuses treatment only on the resected tumor cavity and a surrounding small margin of normal breast tissue. Multiple PBI delivery techniques have evolved, spanning from 1 day to 1 week of twice-daily treatments, based on the rationale that the majority of recurrences occur in close proximity to the original site of disease. Two randomized trials, as well as a multitude of institutional and registry trials, have published early outcomes data, and multiple randomized trials are ongoing. Early data suggest that in the properly selected patient, efficacy and toxicity outcomes are similar to those of standard treatment. To guide clinicians in choosing the most appropriate patients, the American Society for Radiation Oncology issued a consensus statement defining patients who were “suitable,” “cautionary,” and “unsuitable” for PBI.
In the most significant deescalation of therapy, omission of radiotherapy has been evaluated in certain subgroups. Historically it has been difficult to define a subgroup of patients in which the addition of radiotherapy did not result in a significant reduction in in-breast tumor recurrence. However, recently the Cancer and Leukemia Group B reported their data evaluating treatment of women older than 70 years with lumpectomy followed by tamoxifen alone versus lumpectomy, radiotherapy, and tamoxifen. Although a statistically significant decrease in local recurrence was noted, the overall rate of breast tumor recurrence was quite low and the major cause of mortality was unrelated to breast cancer.
Although postmastectomy radiotherapy has historically been reserved for patients with more advanced primary disease, several series suggest that patients with aggressive tumor biology (HER2+ or triple negative) have higher risks of locoregional recurrence. Investigators from China recently reported a significant increase in overall survival (90.4% vs 78.7%) in patients with triple-negative breast cancer receiving adjuvant chemotherapy and radiotherapy versus those receiving only chemotherapy after mastectomy. These data require confirmation in prospective cohorts with strict eligibility criteria and modern chemotherapy.
Medical oncology
ESBC is a heterogeneous group of related disorders to which systemic therapy is tailored based, in part, on the biological or biomarker differences between subtypes, for example, estrogen and/or progesterone receptor positive or negative and HER2 overexpressed/amplified or not. Broadly speaking, systemic therapies can be grouped together as hormonal therapies, chemotherapy, or targeted therapies such as monoclonal antibodies or tyrosine kinase inhibitors. In very general terms, the hormone receptor–positive subtype is treated with endocrine treatment, although chemotherapy is also effective in most cases. Chemotherapy, but not endocrine therapy, is used in treating hormone receptor–negative breast cancers. Anti-HER2 therapies are used in tumors that overexpress HER2, typically in combination with chemotherapy and/or endocrine therapy, depending on the tumor’s hormone receptor status.
Tamoxifen, a selective estrogen receptor modulator, has the longest use as a hormonal therapy and, therefore, has the most well understood toxicity profile including thromboembolic phenomenon and endometrial cancer. The aromatase inhibitors (AIs) include the nonsteroidal AIs such as anastrozole and letrozole and the steroidal AI, exemestane. Though reserved for postmenopausal settings, these agents are thought by many to be slightly more effective than tamoxifen in treating postmenopausal hormone receptor–positive breast cancer. While demonstrating no increased risk of endometrial cancer or thrombotic phenomenon, the AIs decrease the level of systemic estrogen, resulting in exacerbation of menopausal symptoms, loss of bone density, and an adverse effect on the cholesterol profile. No convincing data support that one aromatase inhibitor is more effective than another. Other endocrine therapies including fulvestrant, megesterol, estrogen, androgens, and corticosteroids are generally reserved for patients with advanced hormonally sensitive disease. Gonadotropin-releasing hormone agonists, which induce medical menopause, can also be effective in premenopausal women. Adherence to endocrine therapy is a major challenge in the long-term management of breast cancer. Indeed, studies have suggested that up to 50% of patients discontinue endocrine therapy before completing 5 years.
The most effective chemotherapeutic agents for breast cancer are the anthracyclines, including doxorubicin and epirubicin, and the taxanes, including paclitaxel and docetaxel, although many other agents are approved for the treatment of breast cancer. Short-term toxicities of therapy include alopecia, myelosuppression (which increases the risk of potentially life-threatening infection), nausea and vomiting, neurotoxicity, and fatigue, among others, depending on the agent. Possible long-term side effects include left ventricular dysfunction, primarily from anthracyclines, amenorrhea, and infertility, cognitive dysfunction and other cancers, including myelodysplastic syndrome; and leukemia.
For tumors in which there is amplification of HER2, anti-HER2 therapies improve long-term outcome. Approved anti-HER2 therapies now include the recombinant humanized monoclonal antibodies trastuzumab and pertuzumab and the small-molecule tyrosine kinase inhibitor, lapatinib. An antibody chemotherapy conjugate, trastuzumab-emtansine (T-DM1), has demonstrated encouraging results in patients with advanced disease. Only trastuzumab is approved at this time in patients with ESBC. The monoclonal antibody bevacizumab inhibits angiogenesis and has been shown to have some efficacy in the treatment of metastatic breast cancer. Its toxicity profile, which includes hypertension, thromboembolism, and bleeding, among others, limits its potential in the adjuvant setting.
The purpose of adjuvant systemic therapy is to prevent recurrent disease, prolong disease-free survival, and increase survival rates. All patients with ESBC should meet with a medical oncologist to review the available diagnostic and prognostic information and discuss the need for adjuvant systemic therapy, as well as the appropriate choice of therapy if indicated. Any recommendation should be based on a discussion of the estimated risk of disease recurrence based on tumor type and stage and the potential benefit and harms associated with available systemic therapies. Endocrine therapy, with tamoxifen or an AI, decreases the risk of recurrence of breast cancer and death in women whose breast cancer is hormone receptor positive. Guidelines from ASCO recommend that adjuvant endocrine therapy for postmenopausal women with ESBC include an AI at some point during adjuvant therapy, either as up-front therapy or as sequential treatment after tamoxifen. Although chemotherapy may further reduce the risk of recurrence or death in ESBC, the proportional reduction is generally less in women with hormone receptor–positive disease than that seen with hormonal therapies. As not all patients in this setting appear to benefit from the addition of systemic chemotherapy, predictive measures of chemotherapy benefit are being explored, including gene expression profiling such as the 21-gene assay Oncotype DX, the Amsterdam 70-gene profile Mammaprint, and the Rotterdam 76-gene signature. The most widely used of these is Oncotype DX, which reports a recurrence score (RS) associated with an estimate of distant recurrence-free survival. Available data suggest that tamoxifen-treated patients derive little or no benefit if their tumor is associated with a low RS (Oncotype DX RS <18), whereas they are likely to derive considerable benefit from adjuvant chemotherapy in addition to tamoxifen if a high RS is found (>31). The relative benefit of chemotherapy in patients whose tumor has an intermediate-risk Oncotype DX RS is unclear and is the subject of a randomized controlled trial of hormonal therapy with or without chemotherapy (TAILORx). Although preliminary results suggest that Oncotype DX is prognostic and may be predictive of chemotherapy benefit in patients with positive lymph nodes, the level of risk in this group of patients is sufficiently high to warrant consideration of chemotherapy at present.
Although the choices of chemotherapy regimens for patients with hormone receptor–negative tumors are similar to those for patients with hormone receptor-positive disease, the incremental benefit of adjuvant chemotherapy is generally greater, while hormonal therapy has no role to play in the management of hormone receptor–negative disease. The decision as to whether to treat ESBC patients with chemotherapy is based on the estimated risk of cancer recurrence balanced by the estimated risk of toxicity from treatment. In patients with a low risk of recurrence and for those with a relative contraindication to anthracyclines, a combination of docetaxel and cyclophosphamide (TC) is often used, based on data from a single controlled clinical trial. In patients at greater risk of disease recurrence who have no contraindication, an anthracycline is often combined with a taxane along with cyclophosphamide. In elderly patients and those with serious medical comorbidities, a more nuanced discussion of available chemotherapy regimens and the associated benefit and harms is warranted. However, available evidence supports the use of standard chemotherapy regimens in healthy older women with few competing comorbidities.
Adjuvant trastuzumab added to adjuvant systemic chemotherapy has demonstrated significant improvements in progression-free and overall survival in patients with HER2-positive ESBC. Although trastuzumab is relatively safe, significant declines in cardiac function occur in approximately 20% of patients, and regularly scheduled monitoring of cardiac function is necessary.
Neoadjuvant or primary systemic therapy can be used in cases where decreasing the tumor size would allow for a better cosmetic result or more successful surgery. Although neoadjuvant chemotherapy is advantageous in patients with large tumors and provides an early assessment of chemotherapy responsiveness, no impact of such chemotherapy on patient survival has been demonstrated. Chemotherapy regimens used are similar to those used in the adjuvant setting, with response rates to modern chemotherapy reaching 60% to 70% and pathologic complete response rates ranging from 10% to 30%. Response to chemotherapy, especially response in the lymph nodes, is associated with a better prognosis. In patients with hormone receptor–positive tumors, neoadjuvant endocrine therapy, most notably with an aromatase inhibitor, can be effective and may also be used to answer research questions about tumor biology. Treatment is continued for a minimum of 3 months in the absence of tumor progression, although a maximum response may not be seen for 6 to 12 months.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree