Order
Family
Genus
Species causing human disease
Mucorales
Mucoraceae
Lichtheimia
L. corymbifera
Apophysomyces
A. elegans
Mucor
M. circinelloides, M. ramosissimus, M. racemosus,
M. hiemalis, M. rouxianus
Rhizomucor
R. pusillus
Rhizopus
R. oryzae (R. arrhizus),
R. microsporus var rhizopodiformis
Cunninghamellaceae
Cunninghamella
C. bertholletiae, C. echinulata
Mortierellaceae
Mortierella
(animal pathogens)
Saksenaceae
Saksenaea
S. vasiformis
Syncephalastraceae
Syncephalastrum
S. racemosum
Thamnidaceae
Cokeromyces
C. recurvatus
The Mucorales are characterized in culture by broad nonseptate, or sparsely septate, hyphae and by the presence of sporangiophores supporting sporangia, which contain sporangiospores. Cunninghamella berthollettiae is characterized by sporangiola rather than sporangiospores. During sexual reproduction in culture, zygospores may be produced. The Mucorales are characterized, in tissue by the formation of wide, ribbon-like, hyaline, aseptate (coenocytic) or sparsely septate hyphae with wide-angle (approximately 90°) branching. The substantial differences among these and other structures allow organisms to be diagnosed by genus and species in the mycology laboratory [3].
Epidemiology
The Mucorales are ubiquitous in soil and can be isolated from decaying organic matter including hay, decaying vegetation, and a variety of food items. Human infection is usually acquired through inhalation of sporangiospores from environmental sources. Acquisition via the cutaneous or percutaneous route is also common, either through traumatic disruption of skin barriers or with the use of catheters and injections. Less commonly, infection through the gastrointestinal route may occur [ 1, 3, 6, 7]; however, gastrointestinal mucormycosis is relatively common (54 % of total cases reported) in premature neonates [7].
Mucormycosis is approximately 10–50-fold less common than invasive Candida or Aspergillus infections, with a prevalence of 1–5 cases per 10,000 autopsies and an estimated incidence of 1.7 cases per million per year in the USA [9]. A clear male predisposition has been observed, as demonstrated by an approximate 2:1 male to female ratio among cases [1]. Unlike other filamentous fungi, targeting mainly immunocompromised patients, the Mucorales cause disease in a wider and more heterogeneous population.
The most common underlying condition for development of mucormycosis is diabetes—both type I and type II. A significant proportion of these patients will present with ketoacidosis, while, in others, mucormycosis may even present as the diabetes-defining illness. Other significant underlying conditions include the presence of hematological malignancy, solid organ or hematopoietic stem cell transplantation (HSCT), deferoxamine therapy, and injection drug use [1, 6]. During the past three decades, the percentage of pediatric and adult patients with hematological malignancy, solid organ transplantation or HSCT, and injection drug use among all cases of mucormycosis has significantly increased [1, 10, 11]. In the aforementioned groups of hematological patients and transplant recipients, factors associated with this infection have been reported to include prolonged neutropenia, corticosteroid use, and graft-versus-host disease (GvHD) [6, 11]. Less commonly, the Mucorales may cause invasive disease, in the presence of renal failure, diarrhea, and malnutrition, in low-birth-weight infants and human immunodeficiency virus (HIV) patients. Occasionally, mucormycosis has developed in patients with persistent metabolic acidosis secondary to causes other than diabetes [1, 6].
A significant proportion of mucormycosis cases have, as well, been observed in persons with no primary underlying disease at the time of infection. In many of these cases, there was a history of penetrating trauma, surgery, or burn prior to the development of infection [1, 6].
Pathogenesis and Immunology
The epidemiologic profile of mucormycosis cases (patients with diabetes, hematological malignancies or on deferoxamine therapy, transplant recipients) may, in part, be explained by our current understanding of the pathogenesis of these infections. As with other filamentous fungi, an effective immune response, following inoculation of sporangiospores, requires the presence of adequate phagocytic activity of the host effector cells, including tissue macrophages and neutrophils. Pulmonary alveolar macrophages ingest the sporangiospores to inhibit germination, while the neutrophils are involved in hyphal damage [12]. Consequently, the host immune response against the Mucorales may be compromised if phagocytic cells are insufficient in number as in the case of chemotherapy-induced neutropenia, or dysfunctional, as in the case of corticosteroid treatment or diabetes mellitus [3, 12].
Experimental evidence also suggests an important role of iron in the pathogenesis of infections caused by Rhizopus species, whose growth is promoted in the presence of increased iron uptake. Deferoxamine, an iron chelator, has siderophore activity for these fungi, allowing significant increase in iron uptake. Furthermore, the availability of serum iron is increased in the presence of acidic pH, suggesting an additional mechanism for the development of mucormycosis, in patients with diabetic ketoacidosis [4, 12].
An almost universal feature in infections, caused by the Mucorales, is the presence of extensive angioinvasion associated with thrombosis and ischemic necrosis [3, 13]. This is likely an important mechanism by which these organisms survive antifungal therapy since adequate blood supply is necessary for the delivery of antifungal agents. Recent data also have demonstrated the ability of R. oryzae sporangiospores or hyphae to adhere to subendothelial matrix proteins and human endothelial cells [4, 13]. Pregerminated sporangiospores of R. oryzae are able to damage endothelial cells in vitro, following adherence to and phagocytosis by these cells. R. oryzae viability is not required for endothelial cell damage, suggesting that in the setting of established infection even fungicidal therapy may not prevent subsequent tissue injury [13].
Clinical Manifestations
The clinical manifestations of human infection caused by the Mucorales can be classified as sinus disease, localized or extended to the orbit and/or brain, pulmonary, cutaneous, gastrointestinal, disseminated, and miscellaneous infection [1, 14]. Some of these manifestations may occur with increased frequency in patients with certain underlying conditions (Table 13.2) [1, 6]. However, this is not always the case and mucormycosis, in these patient groups, may still present with any of the above patterns.
Table 13.2
Predominant site of Mucorales infection according to the patient’s underlying condition
Underlying condition | Predominant site of infection |
|---|---|
Diabetes | Sinuses |
Hematological malignancy | Pulmonary and sinuses |
Solid organ transplantation | Pulmonary |
Bone marrow transplantation | Pulmonary and sinuses |
Deferoxamine therapy | Pulmonary, sinuses, disseminateda |
Injection drug use | Cerebral |
No underlying condition | Cutaneous |
Paranasal Sinus Infection
Paranasal sinus disease may be confined to the sinuses or may infiltrate the orbit (sino-orbital) and/or the brain parenchyma (rhinocerebral). This form represents approximately two thirds of all cases of mucormycosis in diabetic patients [1]. The infection originates in the paranasal sinuses following inhalation of sporangiospores. Initial symptoms may suggest sinusitis and include sinus pain, discharge, soft tissue swelling, and perinasal cellulitis/paresthesia. Fever is variable and may be absent in up to half of the cases [4, 14, 15]. The tissues involved become red, violaceous, and finally black, as vascular thrombosis leads to tissue necrosis. A blood-tinged nasal discharge may be present. In sinus disease, nasal endoscopy may show black necrotic crusts on the nasal septum and turbinates; in the early phases, the mucosa may still look pink and viable [4]. We refer to these necrotic ulcers along the nasal mucosa or turbinates as “sentinel eschars,” as they may represent an early phase of infection or may be more amenable to biopsy than a deep maxillary sinus infection.
Extension of the infection to the mouth may produce painful necrotic ulcerations in the hard palate. Extension into the periorbital area, and ultimately the orbit, may be manifested by periorbital edema, lacrimation, chemosis, and proptosis. Subsequent ocular or optic nerve involvement may be suggested by pain, diplopia, blurring, or loss of vision. Alteration of mental status and cranial nerve palsies may signify invasion of the central nervous system. Occasionally, thrombosis of the cavernous sinus or the internal carotid artery may follow, with resultant neurological deficits, while dissemination of the infection also may occur [4, 6, 15, 16].
Pulmonary Infection
Pulmonary disease is most commonly observed in patients with hematological malignancies, solid organ, or HSCT recipients, and those receiving deferoxamine treatment [1]. Not infrequently it may occur with concomitant sinus disease (sinopulmonary infection) [17]. Lung involvement may be manifested as infiltrates, consolidation and solitary, nodular, or cavitary lesions (Fig. 13.1) [18, 19]. Fungal invasion of the pulmonary vessels may result in thrombosis and subsequent infarcts in the lung parenchyma (Fig. 13.2). Angioinvasion may also lead to intraparenchymal bleeding or even hemoptysis, which can be fatal if major vessels, such as the pulmonary artery, are involved. Extension of the infection to the chest wall, pericardium, myocardium, mediastinum, and diaphragm has been described [4, 6, 18]. A predilection for the upper lobes has been reported; however, any part of the lung may be involved, and bilateral disease is not uncommon [18]. Recent studies of the presenting signs and symptoms are nonspecific and include fever, cough, chest pain, dyspnea, hemoptysis, tachypnea, crackles, decreased breath sounds, and wheezing [4, 18, 19]. Endobronchial findings include stenosis or airway obstruction, erythematous mucosa, fungating or polypoid mass and, less often, granulation tissue, or mucosal ulceration [18].
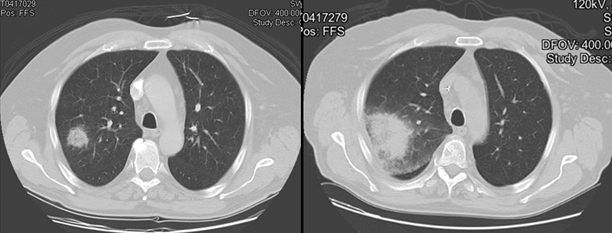
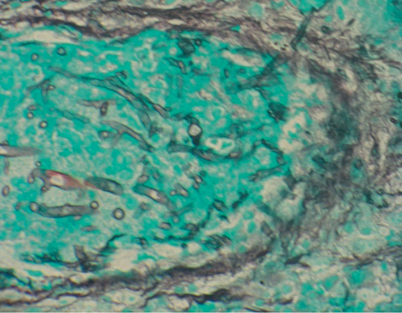

Fig. 13.1
Thoracic CT scan of profoundly neutropenic patient with pulmonary mucormycosis demonstrates rapid evolution of pulmonary nodule to involve the pleural surface and to manifest a halo sign at the interface with radiologically normal lung. The two scans are separated by 5 days. CT computerized tomography

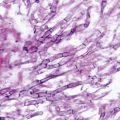
Fig. 13.2
Histopathology of pulmonary mucormycosis in this figure is characterized by broad nonseptate ribbon-like hyphae with non-dichotomous branching invading a pulmonary blood vessel. The specimen was obtained from the lung lesion seen on CT scan in Fig. 13.1. CT computerized tomography
Cutaneous Infection
Cutaneous mucormycosis is often observed in individuals with no underlying condition as a result of infection of a preexisting lesion, such as skin trauma or burn [20]. Alternatively, it may occur in the context of disseminated disease or extensive local infection in immunocompromised hosts [1, 3, 21]. In the case of primary cutaneous inoculation, the lesion appears acutely inflamed with redness, swelling, induration, and frequent progression to necrosis. Extensive local invasion may occur involving the adjacent subcutaneous fat, muscle, bone tissues, and facial layers (Fig. 13.3). When cutaneous disease is the result of disseminated infection, it usually presents as nodular subcutaneous lesions that may ulcerate [3, 6, 21].
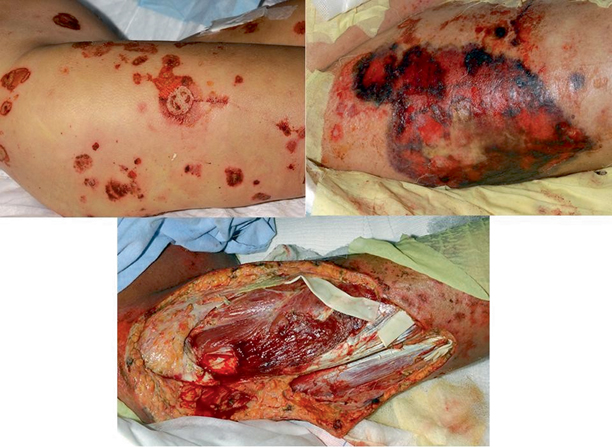

Fig. 13.3
Development of mucormycosis in the skin and subcutaneous tissues of the right lower extremity in a patient with cutaneous T cell lymphoma. The top-left panel depicts the lesions of cutaneous T cell lymphoma, which were possibly infected by direct inoculation. The top-right panel reveals the extensive necrosis and destruction of soft tissue caused by the rapidly invading hyphae. The entire region was anesthetic to any tactile or pressure stimuli. The bottom panel demonstrates the soft tissues following extensive surgical debridement to resect infected skin, fascia, and muscle
Gastrointestinal Infection
Gastrointestinal disease is rare, occurring mainly in malnourished patients and premature neonates, where it can present as necrotizing enterocolitis [1, 7, 22]. After ingestion of the sporangiospores, fungal invasion of the mucosa, submucosa, and vascular structures of the gastrointestinal tract may occur, often resulting in necrotic ulcers, rupture of the intestinal wall, and peritonitis. Symptoms are nonspecific, including fever, abdominal pain, distention, vomiting, and gastrointestinal hemorrhage [3, 4].
Disseminated Infection
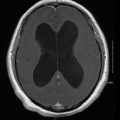
Disseminated infection refers to involvement of at least two non-contiguous sites and is commonly observed in patients receiving deferoxamine therapy [1]. Dissemination occurs through the hematogenous route and may originate from any of the above sites of primary infection; although, it seems to be more frequently associated with lung disease. The most common site of dissemination is the brain but other organs may also be involved [4, 23].
Other Infection
Diagnosis
As infections caused by the Mucorales, in humans, may be rapidly fatal, timely diagnosis is crucial to avoid treatment delay. While confirmation of the diagnosis and species identification of the causative organism should be pursued, treatment should be initiated as soon as the diagnosis is suspected, due to the severity of these infections.
Currently, the diagnosis of mucormycosis relies on a constellation of the following: high index of suspicion, assessment of presenting signs and symptoms, imaging studies, cultures of clinical specimens, and histopathology (Fig. 13.4).
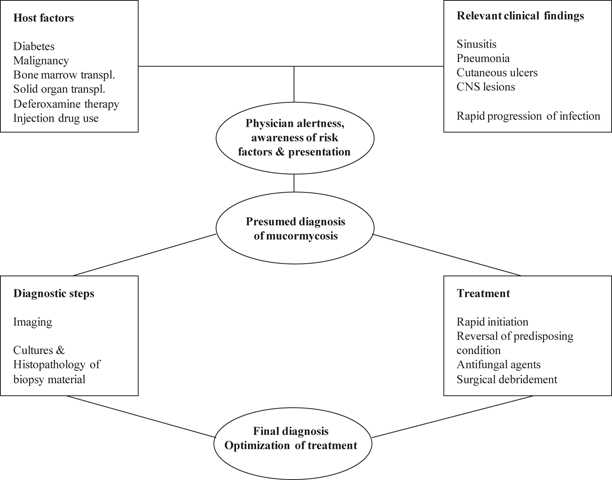

Fig. 13.4
Diagnosis and management of mucormycosis
Clinical Assessment
The high index of suspicion should be based on the knowledge of the underlying conditions that predispose to mucormycosis and the usual presentation of the infection in each of these conditions (Table 13.2). Nevertheless, less common manifestations of the disease should not be excluded. A common scenario is the development of mucormycosis in oncological patients or transplant recipients who are receiving antifungal therapy for prophylaxis or treatment of other opportunistic fungal infections, such as invasive aspergillosis. If antifungal agents, being administered to the patient, are not active against the Mucorales (including, fluconazole, voriconazole, and the echinocandins), then clinical deterioration or appearance of new signs and symptoms in these patients should alert the clinician to the possibility of mucormycosis [17].
Most of the signs and symptoms that are associated with the clinical manifestations of mucormycosis are nonspecific. However, their diagnostic significance may increase if they are interpreted in relation to the patient’s underlying condition. For example, the development of sinusitis in a leukemic or diabetic patient should raise the suspicion of mucormycosis. Other findings have probably greater specificity for this infection, such as the presence of blood-tinged nasal discharge or necrotic eschars in the hard palate. In addition, the presence of hemoptysis in a susceptible host is consistent with angioinvasion and should raise the possibility of mucormycosis [18]. An alarming sign should also be the rapid spread of the infection. Finally, even after the diagnosis has been made, careful periodic clinical assessment should be performed in order to detect progression of the disease. For example, in a patient with pulmonary mucormycosis, palpation of the skin for subcutaneous nodules and neurological evaluation for changes in mental status and focal neurological signs should be repeatedly performed in order to detect dissemination to the skin and brain, respectively.
Diagnostic Imaging
Imaging studies are helpful in assessing the burden of the disease, involvement of adjacent tissues, and response to treatment. They are also helpful in guiding more invasive procedures to obtain biopsy specimens for histopathology and culture [24]. Although imaging findings may be suggestive of mucormycosis in the appropriate clinical setting, they are not sufficiently specific to establish the diagnosis. In sinus disease, computerized tomography (CT) detects subtle mucosal thickening or bony erosions of the sinuses, but it is less sensitive than magnetic resonance imaging (MRI) for the detection of extension of the infection to the soft tissues of the orbit [14, 25]. In the case of pulmonary disease, high-resolution CT is more sensitive than chest radiograph for early diagnosis of the infection, and can, more accurately, determine the extent of pulmonary involvement. Radiographic features consistent with pulmonary mucormycosis include nodular infiltrates, pleural effusions, cavity, consolidation, and reverse halo sign (Fig. 13.1). The air crescent and halo signs, which are recognized radiologic features of invasive aspergillosis, have been reported as well for mucormycosis, while the reverse halo sign in neutropenic patients may have more specificity for pulmonary mucormycosis [18, 19, 26]. In patients with pulmonary mucormycosis, the presence of an air crescent sign seems to be associated with increased risk for massive hemoptysis [18]. Another suggestive finding could be the expansion of a mass or consolidation across tissue planes, in particular, towards the great vessels in the mediastinum [4, 27]. In the case of cutaneous disease, MRI is superior to CT scan for assessment of extension of the infection to the adjacent soft or bone tissues.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree