Although invasive aspergillosis is the most common invasive mold disease (IMD), mucormycosis and other non- Aspergillus opportunistic mold infections are increasingly associated with significant morbidity and mortality among highly immunocompromised patients. Early clinical suspicion is critically important to accurately distinguish, diagnose, and appropriately treat these life-threatening infections. Their relative rarity compared with other infections in these patient populations makes diagnosis and treatment challenging because of the lack of large-scale available data; therefore current clinical outcomes remain far from ideal.
Mucormycosis
Mucormycosis refers to IMD caused by members of the order Mucorales. “Mucormycosis” is now preferred to the historical term “zygomycosis” owing to an updated understanding of fungal phylogenetic relationships. It is the second most common IMD in immunocompromised hosts, after invasive aspergillosis.
Epidemiology and risk factors
Causative genera of mucormycosis are listed in Box 25.1 . Organisms within the genera Rhizopus, Mucor, and Lichtheimia (formerly Absidia ) account for the majority of reported cases. Organisms causing mucormycosis are ubiquitous in the natural environment. Spores can be inhaled into the upper and/or lower airways, inoculated at sites of skin trauma, or rarely, ingested via the gastrointestinal tract. Disease develops primarily in hosts with significant impairment of innate and/or cellular immunity.
Rhizopus
Mucor
Rhizomucor
Actinomucor
Lichtheimia
Cunninghamella
Apophysomyces
Saksenaea
Syncephalastrum
Cokeromyces
Major predisposing factors across multiple types of immunocompromised populations include profound and prolonged neutropenia and high-dose corticosteroid exposure. Additionally, iron overload, hyperglycemia, and ketoacidosis increase risk for mucormycosis even in the absence of other immunosuppressive conditions and can further compound risk when they occur in transplant recipients and oncology patients.
Increasing incidence and breakthrough infections.
The overall incidence of mucormycosis-related hospitalizations in the United States doubled from 1.7 per million in 2000 to 3.4 per million persons in 2013. Breakthrough infection in patients receiving voriconazole, which has anti- Aspergillus activity yet no activity against mucormycosis, has been noted more often among patients with mucormycosis than controls and patients with other IMDs. , Although there may be some selective effect favoring emergence of non- Aspergillus IMD in patients receiving mold-active antifungal therapy, there has also been substantial growth in the at-risk population and improved survival from other opportunistic infections. For example, some studies have found a stable incidence of mucormycosis over time among hematopoietic stem cell transplant (HSCT) recipients, but an increase in the absolute number of cases corresponding to patients who receive transplants. , , Signs or symptoms concerning for an IMD in a patient already receiving a mold-active antifungal agent without mucormycosis coverage (e.g., voriconazole) should increase suspicion for mucormycosis or another rare, potentially antifungal-resistant IMD. Additionally, it is important to maintain consideration of invasive aspergillosis, especially an azole-resistant isolate or an azole-resistant species (e.g., Aspergillus calidoustus ) in the differential diagnosis for breakthrough IMD. Breakthrough IMD could occur because of intrinsic or acquired antifungal resistance, suboptimal antifungal exposure owing to inadequate dosing, nonadherence, high inoculum burden, or host immune factors. Table 25.1 summarizes reported breakthrough IMDs on various antimold prophylaxis agents.
| Antifungal Agent/Class | Predominant Reported Breakthrough Invasive Mold Disease | Other Breakthrough Infections Reported |
|---|---|---|
| Voriconazole | Mucormycosis | Aspergillosis |
| Fusariosis | ||
| Penicilliosis | ||
| Scedosporiasis | ||
| Acremonium infection | ||
| Posaconazole | Aspergillosis | Mucormycosis |
| Fusariosis | ||
| Scedosporiasis | ||
| Penicilliosis | ||
| Rasamsonia (Geosmithia) argillacea infection | ||
| Isavuconazole | Mucormycosis Aspergillosis c | Aspergillosis Fusariosis Scedosporiasis |
| Itraconazole | Aspergillosis | Fusariosis Mucormycosis Scedosporiasis |
| Echinocandins | Aspergillosis | Mucormycosis Fusariosis Exserohilum infection Hormographiella aspergillata infection |
| Amphotericin B deoxycholate or lipid formulation of amphotericin B | Aspergillosis | Undetermined etiology (probable pulmonary invasive mold disease) |
a Reports based primarily on data from adults with hematologic malignancy and/or hematopoietic stem cell transplantation.
b Includes primary or secondary prophylaxis.
c Based on 2 studies only; one reported mucormycosis most commonly and the other reported aspergillosis most commonly.
Hematopoietic stem cell transplant.
Table 25.2 summarizes the key epidemiologic features of mucormycosis in HSCT recipients, solid organ transplant (SOT) recipients, and oncology patients. The majority of data are derived from adult studies. Among HSCT recipients in the Transplant-Associated Infection Surveillance Network (TRANSNET) study from 2001 to 2006, mucormycosis represented 77 (8%) of 983 invasive fungal disease (IFD) cases, whereas invasive aspergillosis represented 43% of IFDs. Mucormycosis is more likely to occur in allogeneic HSCT recipients than autologous HSCT recipients. The median time of onset of mucormycosis in the TRANSNET study was 135 days after transplant versus 99 days after transplant for invasive aspergillosis.
| Feature | Hematopoietic Stem Cell Transplant | Solid Organ Transplant | Oncology |
|---|---|---|---|
| Incidence estimates | Cumulative incidence during first year after transplant: 0.29% in autologous and allogeneic cohort (TRANSNET) 0.60% in allogeneic only cohort (CIBMTR) | Cumulative incidence during first year after transplant: 0.07% (TRANSNET) | 72.0 mucormycosis-related hospitalizations per 100,000 hematologic malignancy hospitalizations |
| Timing of onset | Median, 4.4 months after transplant (TRANSNET) Median, 75 days after transplant (CIBMTR) | Median, 5 months after transplant, earlier in liver transplant recipients | Median, 8.8 months after diagnosis |
| Risk factors for disease | Allogeneic transplantation Unrelated donor Acute graft-versus-host disease grade II-IV Prior aspergillosis | Lung transplantation Liver transplantation Recent organ rejection episode Diabetes mellitus Renal failure before transplant | Hematologic malignancy, particularly acute myelogenous leukemia Active malignancy Prolonged (>7 days) neutropenia |
| Mortality/case-fatality rate estimates | 72% at 1 year after diagnosis of mucormycosis (TRANSNET) 85% at 1 year after diagnosis of mucormycosis (CIBMTR) | 38% at 90 days | 52% during course of mucormycosis (follow-up period undefined) |
Solid organ transplant.
Among primarily adult SOT recipients in the TRANSNET study, mucormycosis represented only 2% of all IFDs versus 19% caused by invasive aspergillosis. Compared with other types of SOT recipients, liver transplant recipients present earlier after transplant and have a higher frequency of disseminated disease; their risk is hypothesized to be related to iron overload. , Mucormycosis is rare among pediatric SOT recipients.
Oncology.
Hematologic malignancy (with or without HSCT) is the most common underlying condition reported among adult and pediatric patients with mucormycosis, accounting for 45 to 60% of cases in large series. Patients undergoing therapy for hematologic malignancy often share multiple concurrent risk factors for mucormycosis.
Prognosis and modifying factors.
Mucormycosis is a highly fatal disease; however, specific mortality estimates vary widely depending on the clinical population and duration of follow-up. A recent pediatric case series reported a case fatality rate of 33.3% at last follow-up. Risk factors for death include disseminated disease, hematologic malignancy, HSCT, monocytopenia at diagnosis, and lymphopenia at diagnosis. , , In some studies, higher mortality has been noted with infection caused by Cunninghamella species. Favorable prognostic factors include localized cutaneous disease, surgical resection of disease, early amphotericin B–based therapy, and neutrophil recovery. , , ,
Clinical manifestations
Table 25.3 lists the major clinical syndromes of mucormycosis with their relative frequency in transplant recipients and oncology patients. The clinical manifestations of mucormycosis are nonspecific and similar to those of other IMDs. Individual patients may present with subtle or few symptoms initially, requiring a high index of suspicion from the clinician. Pulmonary infection is the predominant clinical syndrome in transplant recipients and oncology patients, as opposed to rhinocerebral infection in patients with diabetic ketoacidosis and cutaneous infection in patients in whom mucormycosis develops after trauma. Disseminated mucormycosis refers to involvement of 2 or more noncontiguous sites. Regardless of the site of disease, vascular invasion is a characteristic feature of disease and can lead to thrombosis, septic emboli, and rapid progression.
| Syndrome | Percentage of Cases a | Clinical Manifestations |
|---|---|---|
| Pulmonary | 50-60 | Fever, cough, chest pain, dyspnea, hemoptysis |
| Rhinocerebral | 15-30 | Facial swelling, pain, proptosis, headache, nasal congestion, nasal discharge, necrotic lesions of palate or nasal septum, cranial neuropathies With brain involvement: seizure, stroke, focal neurologic deficit(s), encephalopathy |
| Cutaneous | 10-20 | Erythematous, indurated lesion, progression to ulcer, then necrotic eschar |
| Gastrointestinal | <5 | Abdominal pain, nausea, vomiting, gastrointestinal bleeding, obstruction, perforation |
| Disseminated | 15-25 | Variable based on site of dissemination |
a Based on series in hematopoietic stem cell transplant, solid organ transplant, and oncology patients. , , ,
Pulmonary mucormycosis.
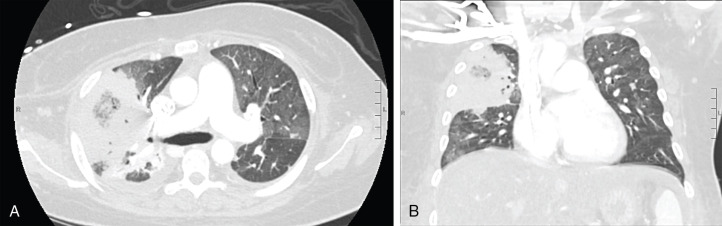
The presenting symptoms and signs of pulmonary mucormycosis are similar to those of other pulmonary IMDs. Radiographic findings can include nodules, consolidation, cavitary lesions, and/or wedge-shaped lung infarcts. Despite the clinical similarities, case series evaluating radiographic findings of mucormycosis in patients with hematologic malignancies have identified certain findings more frequently in patients with pulmonary mucormycosis as opposed to pulmonary aspergillosis. These include multiple pulmonary nodules (>10), pleural effusion(s), and the reverse halo sign. The reverse halo sign ( Fig. 25.1 ) is a focal area of ground-glass opacity surrounded by a ring of consolidation; among patients with hematologic malignancies it is strongly associated with mucormycosis. However, there are other potential etiologies of the reverse halo sign, so it should be interpreted based on pretest probability. The reverse halo sign is uncommon in nonneutropenic patients with mucormycosis.
Rhinocerebral mucormycosis.
Different literature sources refer variably to rhino-orbital, sino-orbital, sinus, rhinocerebral, or rhino-orbito-cerebral mucormycosis. In this chapter we use the term “rhinocerebral mucormycosis” to describe infection involving any of the following structures: the palate, the sinuses, the orbit and any adjacent structures, with or without extension via contiguous or hematogenous routes to the brain. Manifestations depend on the specific sites and the extent of disease involvement. Features that distinguish rhinocerebral mucormycosis from rhinocerebral aspergillosis include a propensity to involve the orbit, involvement of the ethmoid sinuses, and pansinusitis. Concurrent pulmonary and rhinocerebral/sinus involvement should also prompt suspicion for mucormycosis as opposed to aspergillosis.
Cutaneous mucormycosis.
Cutaneous lesions seen in mucormycosis can be primary, developing after localized inoculation at sites of trauma, intravascular catheters or adhesive tape, or secondary owing to hematogenous dissemination from another site of disease.
Other forms of mucormycosis.
The gastrointestinal tract is the least commonly involved primary site of mucormycosis; its manifestations are described in Table 25.3 . One exception is in neonatal disease, but the pathogenesis is likely different than mucormycosis in transplant recipients or oncology patients. Mucormycosis can involve any organ or tissue, either via hematogenous dissemination, deep contiguous extension from the primary focus, or inoculation at sites of trauma or surgery. The brain is one of the most common sites involved via hematogenous dissemination.
Disease prophylaxis
Guidelines for diagnosis and management of mucormycosis have been developed jointly by the European Society for Clinical Microbiology and Infectious Diseases (ESCMID) and the European Confederation of Medical Mycology (ECMM). These guidelines, based primarily on adult data, offer a marginal recommendation for primary prophylaxis with posaconazole during periods of graft-versus-host disease (GVHD) with augmented immunosuppression and during outbreak situations. Use of posaconazole as secondary prophylaxis is recommended during ongoing immunosuppression in patients who have previously been diagnosed with mucormycosis. Guidelines developed for diagnosis and treatment of mucormycosis in patients with hematologic malignancy from the third European Conference on Infections in Leukemia (ECIL-3), and subsequently updated (ECIL-6), do not provide recommendations on primary prophylaxis but support the use of posaconazole for secondary prophylaxis. , Available pediatric dosing data for are limited to children 13 years and older, limiting use of this medication in younger patients. It is important to recognize that patients receiving posaconazole prophylaxis can still develop mucormycosis as a breakthrough infection (see Table 25.1 ).
Diagnosis
The diagnosis of mucormycosis can be challenging and relies on early clinical suspicion and aggressive pursuit of diagnostic samples. A suggested diagnostic approach is outlined in Fig. 25.2 . Multidisciplinary coordination is recommended between transplant and oncology specialists, infectious diseases and surgical specialists, along with clinical pathologists, clinical pharmacists, and microbiologists. Empiric antifungal therapy should be started promptly and concurrently with attempts to establish the diagnosis, because delay in treatment has been associated with increased mortality.
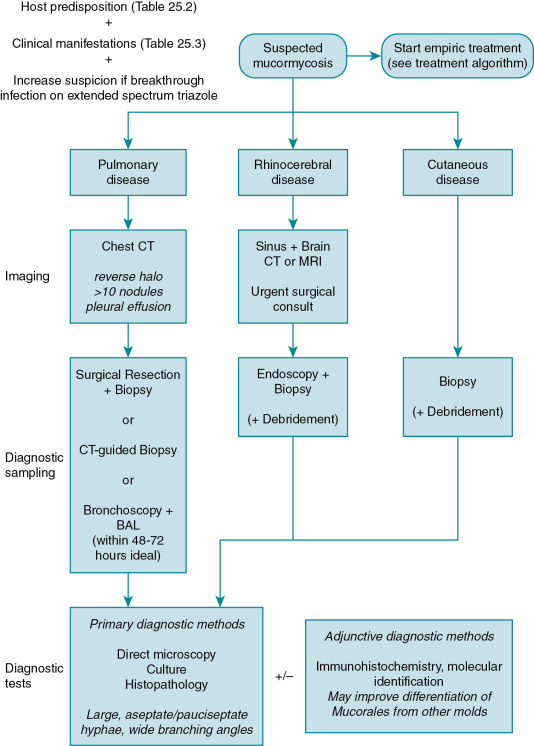
Diagnostic sampling.
Obtaining clinical samples from the affected site(s) is essential as there are currently no standardized diagnostic biomarkers or other well-validated noninvasive tests to diagnose mucormycosis. Results of serum galactomannan and (1,3)-β-D-glucan tests are usually negative in mucormycosis as these antigens are not released or not released in detectable quantities by the Mucorales. The ideal sample for diagnostic evaluation is tissue from the affected site(s) for histologic verification. Tissue samples are more readily obtainable in the setting of rhinocerebral and cutaneous disease but are challenging to obtain from the lungs. Unfortunately, the lungs are the predominant site of mucormycosis in transplant recipients and oncology patients. Recovery of Mucorales from sputum and bronchoalveolar lavage (BAL) samples is low (25% from BAL in one study); however, BAL may be useful in evaluating for other etiologies of pneumonia in an immunocompromised patient, and experts have suggested higher potential yield in the first 48 to 72 hours from symptom onset. Computed tomography (CT)-guided lung biopsy has shown utility in establishing the diagnosis of pulmonary mucormycosis. Correction of coagulopathy and/or transfusion of platelets may be required to reduce bleeding risk before a patient can undergo biopsy. Correcting any identified coagulopathy is of particular importance in this setting given the angioinvasive nature of these pathogens, which may further predispose the patient to bleeding during or after a biopsy procedure. Although clinicians may be reluctant to pursue invasive procedures, the value of an invasive intervention should be emphasized given its influence on antifungal therapy choice (e.g., different antifungal classes for invasive aspergillosis versus mucormycosis), the likelihood for earlier initiation of appropriate directed therapy, and the opportunity for improved source control with removal of necrotic tissue that might diminish antifungal effectiveness.
Primary diagnostic tests.
Direct microscopy of clinical samples with an optical brightener such as calcofluor white can provide early confirmation of the diagnosis. , Causative organisms of mucormycosis can be visualized on histopathology via commonly used tissue stains. Hyphae of Mucorales are large with variable width (6 to 25 μm), irregular, and ribbon-like in appearance, contain few or no septations, and demonstrate wide-angle (90-degree) branching. As histology often offers the first clues to pathogen identification, it is critical to recognize these morphologic characteristics of Mucorales and to identify how they differ from other fungi. For example, hyphae in invasive mucormycosis differ from invasive aspergillosis (regularly septate, smaller hyphae). In some cases, damage to tissue or a paucity of organisms precludes differentiation of Mucorales from other molds via conventional histopathology. Therefore additional methods for identification of the organism are necessary. This can include a constellation of diagnostic testing, including immunohistochemistry, conventional culture, and more contemporary molecular methods. Practitioners should also be aware of the possibility of co-infection with other molds.
Owing to the lack of septations, the organisms are prone to shearing during tissue processing, and culture results may be negative even with organisms visualized on histopathology. Mincing of tissues rather than grinding is recommended to improve recovery in culture. Speciation of the Mucorales is difficult using conventional microbiology methods, but can be improved using adjunctive molecular methods. The ESCMID/ECMM and ECIL guidelines for mucormycosis recommend identification to the genus and species level, if possible, primarily for epidemiologic understanding. It is not clear at this time that identification of the genus and species is important to guide clinical management. Standardized methods of microdilution susceptibility testing for the Mucorales are guided by the European Committee on Antimicrobial Susceptibility Testing and Clinical & Laboratory Standards Institute, but no validated susceptibility breakpoints have been established. Methodologies for determining an epidemiologic cutoff value have been developed and used for common species. These cutoffs can provide a guide to the clinician regarding the relative susceptibility of an organism. However, despite the terminology, epidemiologic cutoffs are not correlates of clinical effectiveness.
Adjunctive and emerging diagnostic tests.
Novel methods show potential to supplement conventional diagnostic methods for mucormycosis. Immunohistochemistry on histopathology samples and molecular detection methods, including polymerase chain reaction–based strategies and matrix-assisted laser desorption ionization time-of-flight mass spectrometry have been demonstrated in small clinical cohorts. Few molecular diagnostic tests are currently available commercially, and there is a lack of standardized approaches. To the extent that these tests are available, they may be useful in providing genus- and species-level identification when Mucorales are detected via conventional methods, in differentiating Mucorales from other molds when histopathologic findings are ambiguous, or improving detection of Mucorales from lower-yield clinical samples such as BAL fluid. When molecular detection methods are used, fresh clinical samples are preferred over formalin-fixed paraffin-embedded samples.
Some of the most promising strategies are those that may establish the diagnosis of mucormycosis without invasive diagnostic sampling. Such strategies include detection of circulating Mucorales DNA in the blood via polymerase chain reaction, detection of Mucorales-specific host immune responses, and detection of a characteristic metabolite signature in exhaled breath. Unbiased pathogen detection via circulating cell-free nucleic acids has also shown potential to detect IMD including mucormycosis. Such methods are promising and may change the future diagnostic strategy for mucormycosis and other IMDs. However, before they are comprehensively validated against conventional diagnostic methods, they should not be considered a replacement for diagnostic biopsy in patients with suspected mucormycosis.
Treatment
Mucormycosis is a rare disease and thus it is difficult to study therapeutic options via robust clinical trials. The only randomized controlled trial of mucormycosis therapy enrolled just 20 patients and focused on adjunctive therapy, largely in patients with diabetes mellitus. Most treatment recommendations are therefore based on observational data, small single-arm clinical trials, animal models, and expert opinion. The treatment algorithm outlined in Fig. 25.3 is based on a synthesis of European and Australian clinical guidelines, expert opinion reviews, and pediatric considerations for drug therapy. , Mucormycosis is life-threatening and can be a rapidly progressive condition, yet favorable outcomes are achievable and most likely to occur when antifungal therapy is combined with surgery and reversal of predisposing conditions. An expeditious and multidisciplinary approach to therapy is recommended.
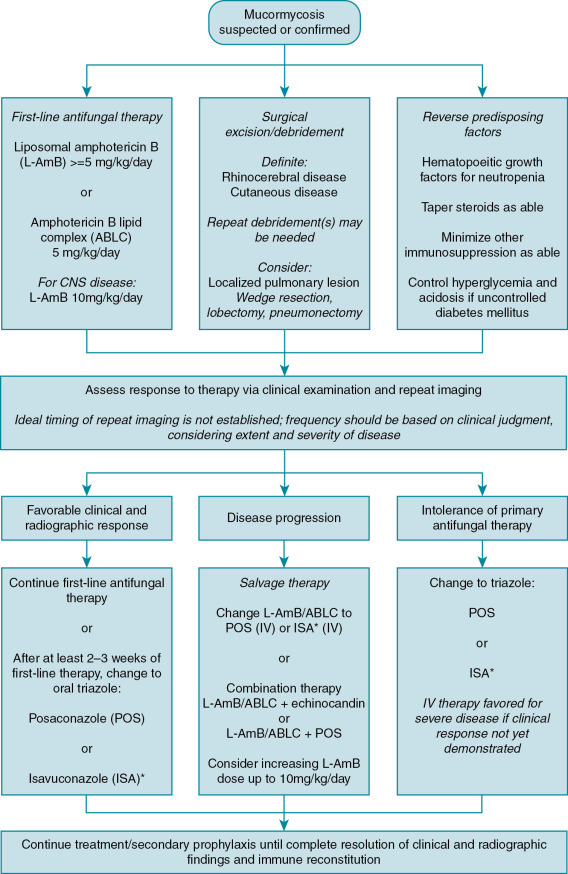
Primary antifungal therapy
Amphotericin B–based monotherapy.
Amphotericin B–based therapy is recommended as first-line treatment of mucormycosis in all age groups based on a preponderance of observational data demonstrating its impact on survival. , Although conventional amphotericin B deoxycholate has been used historically, its use is discouraged outside the neonatal period because of poor tolerability. Among the lipid formulations of amphotericin B, liposomal amphotericin B (L-AmB) is favored, especially for central nervous system (CNS) disease and for patients with renal insufficiency. Amphotericin B lipid complex (ABLC) is also an option. It is important to initiate therapy upon suspicion of mucormycosis, as delay of more than five days from onset of symptoms has been associated with near doubling of mortality. The optimal dose of L-AmB is not well-defined; at least 5 mg/kg per day is recommended. Escalation of the dose up to 10 mg/kg per day increases drug exposure and improves disease response in animal models, but it is not clear that it alters clinical response in humans. A single-arm trial of L-AmB at 10 mg/kg per day in 40 patients showed clinical response rates similar to those reported in observational literature, but creatinine doubling occurred in 40% of participants. Nonetheless, clinical guidelines recommend L-AmB at 10mg/kg per day for treatment of CNS mucormycosis, primarily based on animal model and case report data. ,
Triazole monotherapy.
Isavuconazole is an extended-spectrum triazole antifungal now licensed for primary therapy of mucormycosis in adults based on a single-arm trial (VITAL) of 37 patients 18 years and older with mucormycosis, including 21 receiving the drug as primary therapy. The 6-week all-cause mortality of 33% in primary treatment cases was comparable to 39% in amphotericin B–treated matched controls from a registry. A pediatric pharmacokinetic and safety study of isavuconazole is in progress. Given the current lack of pediatric data and limitations in the methodology of the VITAL study, clinicians should use caution in considering isavuconazole for primary therapy for mucormycosis in children. Potential pitfalls reported are breakthrough mucormycosis in patients receiving isavuconazole (see Table 25.1 ), and high minimum inhibitory concentrations to isavuconazole among some causative pathogens, particularly Mucor circinelloides . No other monotherapy regimens, including posaconazole, have been systematically studied for primary therapy of mucormycosis.
Combination antifungal therapy.
Some experts recommend routine use of combination antifungal therapy with L-AmB and an echinocandin as primary therapy for mucormycosis. The rationale is based on in vitro and animal model studies. Although echinocandins lack activity against the Mucorales when given as monotherapy, their target enzyme, (1,3)-β-D-glucan synthase, is expressed at least by some species, and animal studies indicate they may be beneficial when combined with lipid formulations of amphotericin B. Small retrospective case series have shown better outcomes in patients treated with combination therapy versus monotherapy. The studies are limited by small size, retrospective design, and lack of clear comparability between the combination therapy and monotherapy-treated patients with respect to surgical management or other important factors influencing outcome. Generalizability to transplant and oncology patients is also unclear based on combination therapy data derived from patients with rhinocerebral mucormycosis in the setting of diabetic ketoacidosis.
Larger observational studies incorporating methods to control for confounding, including “natural experiment” analysis based on era of treatment, or propensity score adjustment, have conversely not shown a benefit to combination therapy. , Clinical guidelines for mucormycosis conclude that there is insufficient evidence at this time to recommend primary combination therapy. , Some experts advocate for primary combination therapy based on theoretical benefit with modest impact on cost or toxicity of treatment, but more data are needed to support these conclusions.
Animal studies have shown conflicting results regarding additional benefit of posaconazole when added to lipid-formulated amphotericin B for primary treatment of mucormycosis. Clinical studies supporting this combination for primary therapy are lacking. Some experts recommend this combination as empiric treatment when the etiology of a breakthrough IMD is undetermined. The primary rationale is to broaden the spectrum of empiric treatment in case of a relatively drug-resistant species of Aspergillus, Mucorales, or another breakthrough IMD.
Salvage antifungal therapy.
Responses to primary antifungal therapy in mucormycosis are characteristically poor, slow, and often very dependent on host immunologic status. Even in cases with an ultimate favorable outcome, there may be initial disease progression before improvement (especially if there has been evidence of immune recovery such as resolution of neutropenia), and disease control can take several weeks. Therefore clinicians must resist desires for early transition to salvage therapy. Unfortunately, knowing when to adjust a therapeutic plan can be challenging, and often initial disease progression prompts modification to a salvage therapy approach. Clinical trials of salvage antifungal therapy typically enroll patients considered to have refractory disease after at least 7 days of primary antifungal therapy. Additionally, intolerance of amphotericin B–based therapy, primarily because of nephrotoxicity, may prompt modification of therapy to an alternative agent. Posaconazole has demonstrated favorable effectiveness as salvage therapy for mucormycosis, with response rates of 60% to 80%. It is important to avoid overinterpreting salvage therapy response rates as indicating potential responses to primary therapy. Patients who receive salvage therapy have survived long enough since diagnosis to be eligible for salvage therapy, and they may have benefited already from initial surgery and first-line antifungal therapy. Given favorable data for posaconazole, clinical guidelines recommend it as an option for salvage therapy in patients with refractory disease or life-threatening intolerance of first-line therapy. , Isavuconazole has also been studied in adults for salvage therapy of mucormycosis.
Salvage therapy because of intolerance of primary therapy.
In our treatment algorithm ( Fig. 25.3 ), we suggest differentiation of patients who switch to salvage therapy because of intolerance of primary therapy from those who switch because of disease progression. Changing to posaconazole (or off-label isavuconazole in selected adolescents) is reasonable for clinically stable patients with life-threatening intolerance to amphotericin B–based therapy if they do not show disease progression and do not have CNS disease. Although initial studies of posaconazole used the suspension formulation, the delayed-release tablet formulation is preferred now for patients age 13 years or older owing to improved bioavailability. Intravenous (IV) formulations of posaconazole and isavuconazole are also available and initial use of IV therapy is favored in patients with severe disease. Optimal dosing of posaconazole for younger children (<13 years) is not well-established, and the oral suspension has poor and highly variable bioavailability. Posaconazole therapeutic drug monitoring is recommended, with a suggested target trough of > 1 mg/L based primarily on expert opinion and extrapolation from other invasive fungal diseases.
Salvage therapy because of refractory or progressive disease.
Switching to posaconazole (or off-label isavuconazole in selected adolescents) is an option for salvage therapy in patients with refractory or progressive disease; however, caution should be exercised against early abandonment of guideline-recommended amphotericin B–based therapy. Compared with triazole agents, amphotericin B is active against a wider spectrum of causative agents of mucormycosis. Animal models raise concern for failure of posaconazole against Rhizopus arrhizus (formerly known as Rhizopus oryzae ) and Mucor circinelloides , 2 of the most common causative organisms. The potential gaps in the spectrum with isavuconazole are not well characterized, but breakthrough mucormycosis has been reported in patients receiving isavuconazole. Posaconazole is a P-glycoprotein substrate and thus achieves poor concentration in the CNS. Owing to unknowns and potential limitations with these agents as monotherapy, clinicians may wish to continue L-AmB and add posaconazole or isavuconazole for refractory or progressive mucormycosis. Although there has been concern about antagonism between these agents in other fungal diseases, in vitro data have not supported antagonism when used in combination for Mucorales organisms. Some clinical guidelines have suggested salvage therapy with combination L-AmB and an echinocandin, whereas some experts have offered the suggestion of escalating the dose of L-AmB up to 10 mg/kg per day for refractory disease. Unfortunately, there are limited data to support or refute these recommendations. Likewise, there has been no systematic evaluation of triple-combination therapy, including L-AmB, posaconazole and an echinocandin, but this regimen is sometimes used in clinical practice.
Given the limited evidence for an ideal approach, treatment of the patient with refractory or progressive mucormycosis should be individualized, considering the potential risks and benefits of the therapy based on the patient’s age, comorbidities, and concurrent medications. Clinicians should familiarize themselves with the antifungal agents, their pharmacokinetic characteristics in children, drug interactions and adverse effects, and ideally work closely with a multidisciplinary team to plan and monitor therapy. It is also critical to pursue surgical debridement and reversal of predisposing factors to the extent possible.
Step-down therapy and duration.
Response to therapy should be assessed by clinical evaluation and repeated imaging of the affected site(s). The optimal timing of reassessment and optimal duration of initial therapy are not established. More frequent repeat imaging (weekly) is suggested for patients with severe and/or extensive disease, or those with potential invasion of vital structures such as the thoracic vasculature or CNS. Patients who show a favorable clinical response and have well-controlled localized disease may not require such frequent repeat imaging. Amphotericin B–based primary therapy should be continued until at least a partial response is demonstrated clinically and radiographically; this usually takes several weeks. Patients who demonstrate a response may either continue with amphotericin B–based therapy or switch to oral posaconazole (or off-label isavuconazole, in selected adolescents) for long-term maintenance therapy. Transition to an azole regimen in children younger than 13 years is challenging as there are limited dosing recommendations for these agents in this age group. Therefore continued use of amphotericin B–based therapy even after initial clinical response is favored by some experts. Amphotericin B–based therapy toxicity or intolerance may necessitate use of posaconazole or isavuconazole in these patients. In such situations, guidance from an experienced pediatric pharmacist, along with input from the members of the multidisciplinary team, is important. Guidelines for mucormycosis recommend continuation of antifungal therapy until complete resolution of clinical and radiographic findings, and reconstitution of immune function. , Clinicians should be aware of the potential for recrudescence of mucormycosis in patients receiving oral triazoles. Therapeutic drug monitoring is recommended to ensure adequate posaconazole exposure, with a suggested target trough of more than 1 mg/L. Currently there are no recommendations for therapeutic drug monitoring of isavuconazole, but this may change with additional experience as it did with other agents in the azole class.
Surgical management.
Surgical debridement in combination with antifungal therapy improves survival and is strongly recommended for patients with the rhinocerebral and cutaneous forms of mucormycosis. , The goals of surgery are to remove devitalized tissue that is poorly penetrated by antifungals, to limit local extension of disease, and to potentially prevent hematogenous dissemination. Rhinocerebral mucormycosis is considered a surgical emergency. Repeated debridement is often needed and should be guided based on repeated endoscopic examination and imaging. Input from a surgical specialist with experience in debriding rhinocerebral mucormycosis can further optimize care. Similar to invasive aspergillosis, the role for surgical resection of localized pulmonary lesions via wedge resection, lobectomy, or pneumonectomy is not as well established but should be considered as it may be associated with a survival benefit. ,
Reversal of predisposing conditions and adjunctive therapy.
Measures to reverse predisposing conditions are recommended for all patients with mucormycosis because ongoing immune dysfunction is consistently associated with poor outcomes. Hematopoietic growth factors (granulocyte colony-stimulating factor or granulocyte-macrophage colony-stimulating factor) are recommended to reverse neutropenia. Steroids should be tapered as possible and other immunosuppressive therapies should be reduced. Control of hyperglycemia and acidosis is important for patients with uncontrolled diabetes mellitus.
If the patient is receiving the iron chelator deferoxamine, it should be discontinued because it increases the risk for disseminated mucormycosis. Deferoxamine serves as a siderophore and makes iron available for fungi to use metabolically; other iron chelators do not serve as siderophores and their use has been considered for adjunctive therapy to reduce available iron. A clinical trial (Deferasirox-AmBisome Therapy for Mucormycosis) of the iron chelator deferasirox as adjunctive therapy for mucormycosis showed unexpectedly increased mortality among patients with hematologic malignancy in the deferasirox arm. Although this result could have occurred because of imbalance of baseline risk factors in the 2 treatment arms, the study results have led to recommendation against use of adjunctive deferasirox.
Other adjunctive strategies, predominantly described in case reports, include the use of hematopoietic growth factors to augment immune response in patients without neutropenia, use of granulocyte transfusions in patients with refractory neutropenia and fungal disease, and use of interferon gamma to enhance the immune response. None of these strategies are routinely recommended because of limited supporting evidence. Hyperbaric oxygen therapy has shown benefit in case reports and series of patients with diabetes mellitus and rhinocerebral mucormycosis, but limited data in patients with hematologic malignancy have not supported a benefit and it is not routinely recommended in immunocompromised patients with mucormycosis.
Infection prevention and anticipatory guidance
Mucormycosis can be transmitted as a nosocomial infection in the context of hospital construction activities or contaminated supplies. Procedures for air filtration and recirculation should be used in oncology, hematology, and HSCT wards to limit environmental mold exposure. Given the rarity of mucormycosis, clustering of cases should trigger investigation for a potential nosocomial source. Patients should be counseled to avoid activities with risk for high inoculum exposure to inhaled aerosolized fungal spores, such as construction activities or soil excavation. If such exposure is unavoidable, then a mask may be worn in high-risk areas.
Fusariosis and Scedosporiasis
Fusarium and Scedosporium are genera of hyaline molds that are usually the third and fourth most common IMDs in immunocompromised hosts, after invasive aspergillosis and mucormycosis. The causative species are morphologically similar to Aspergillus and cause a similar spectrum of disease; fusariosis and scedosporiasis can be rapidly progressive and challenging to treat owing to multidrug resistance.
Epidemiology and risk factors
Causative organisms of fusariosis and scedosporiasis are grouped into species complexes encompassing member species that can be differentiated via molecular methods. The majority of human cases of fusariosis are caused by members of the F. solani, F. oxysporum, and F. fujikuroi species complexes, with the F. solani species complex demonstrating greater pathogenicity.
Nomenclature of the organisms causing scedosporiasis can be confusing and has undergone recent changes. The genus name Pseudallescheria applies to the sexual state (teleomorph), whereas Scedosporium applies to the asexual state (anamorph) of these organisms. S. apiospermum was once thought to be the anamorph of Pseudallescheria boydii , but they are now known to be distinct species. The S. apiospermum species complex encompasses S. apiospermum , S. boydii , S. aurantiacum, S. dehoogli, and S. minutispora . The organism formerly known as S. prolificans is now renamed Lomentospora prolificans and is phylogenetically distinct from the Scedosporium species. L. prolificans is categorized by some sources as a dematiaceous (pigmented, melanized) mold rather than a hyaline mold (akin to Aspergillus, Fusarium, and Scedosporium ). However, it is usually grouped clinically with the Scedosporium species and the former nomenclature may be seen in clinical references.
In addition to the usual airborne and cutaneous inoculation routes of acquisition common to other invasive molds, Fusarium can be transmitted via contaminated water sources (e.g., shower heads) and can cause infection associated with IV catheters. Both Fusarium and Scedosporium/Lomentospora species can cause infection in immunocompetent hosts, primarily localized infections such as keratitis or onychomycosis. In immunocompromised patients, Fusarium can disseminate from initially localized infections such as onychomycosis or intertrigo. The major predisposing factors for invasive disease are profound and prolonged neutropenia and severe cell-mediated immunodeficiency.
Although fusariosis and scedosporiasis are generally less common than invasive aspergillosis and mucormycosis, their relative incidence varies geographically. For example, a multicenter study in Brazil identified invasive fusariosis more commonly than invasive aspergillosis in HSCT recipients and patients with hematologic malignancy, and the incidence of invasive fusariosis there increased over 10-fold up to 10 cases per 1000 hospitalizations from 2000 to 2010. ,
Hematopoietic stem cell transplant.
Among HSCT recipients in the TRANSNET study, fusariosis accounted for 3% of IFDs. The estimated incidence among allogeneic HSCT recipients from the Center for International Blood and Marrow Transplant Research registry from 1995 to 2008 was 4.34 cases per 1000 patients who had transplants. The incidence of fusariosis was lower between 2002 and 2008 compared with the 1995 to 2002 period; the difference is hypothesized to be related to voriconazole prophylaxis and empiric therapy in the later period. Identified risk factors for fusariosis in HSCT recipients include history of CMV infection, receipt of an umbilical cord blood transplant (compared with other stem cell sources), receipt of antithymocyte globulin, and hyperglycemia. , Risk factors specific to the development of fusariosis beyond day 40 after transplant include GVHD and prior IMD. Scedosporiasis is less common among HSCT recipients compared with fusariosis, with only 16 cases identified in the TRANSNET study compared with 31 cases of fusariosis and 77 cases of mucormycosis. Specific risk factors for scedosporiasis among HSCT recipients are not well described.
Solid organ transplant.
Among 1208 invasive fungal infections in the TRANSNET study of SOT recipients, there were 6 cases of fusariosis and 11 cases of scedosporiasis, compared with 28 cases of mucormycosis. In a literature review of L. prolificans cases, SOT recipients constituted 8.6% of cases. Scedosporiasis is most common among lung transplant recipients, in whom colonization of the airways can occur before transplantation (particularly in patients with cystic fibrosis) or after transplantation, and may progress to invasive infection. Some centers consider colonization with Scedosporium species to be a contraindication to lung transplantation, given the risk for dissemination and high mortality after transplantation, but this practice varies. Disseminated scedosporiasis can develop in immunocompetent persons after drowning, and transmission of scedosporiasis from SOT donor to multiple recipients has been described in the context of the donor’s death caused by drowning.
Oncology.
Among patients undergoing therapy for cancer, fusariosis primarily occurs in those with hematologic malignancy, especially acute myelogenous leukemia (AML). In Brazil, where fusariosis is a relatively common cause of IFD, the 1-year cumulative incidence of fusariosis in patients with AML or myelodysplastic syndrome was 5.2%. In a single-center study of 44 cases, the most commonly identified risk factors for fusariosis in patients with hematologic malignancy were active leukemia, prolonged and profound neutropenia, and high-dose corticosteroid exposure. Smoking has been identified as a risk factor for fusariosis in adults. Pediatric case series have described fusariosis occurring in children with AML, acute lymphoblastic leukemia, and juvenile myelomonocytic leukemia. , Scedosporiasis is less common overall, but malignancy, primarily hematologic, was the most common underlying risk factor in a review of reported cases of scedosporiasis.
Prognosis and modifying factors.
Fusariosis and scedosporiasis confer high mortality, with the ultimate outcome dependent on the extent of disease, the causative species complex, and the degree to which immune function is reconstituted. A multinational study of fusariosis cases (predominantly in adults, but inclusive of children) showed a 43% survival rate at 90 days between 2001 and 2011, an improvement from 22% at 90 days between 1985 and 2000. Treatment with voriconazole was associated with higher probability of survival; receipt of corticosteroids and lack of neutrophil recovery were associated with lower probability of survival. Disseminated fusariosis with fungemia carries a particularly high case-fatality rate with one series reporting only 6% survival at 6 weeks. Overall mortality resulting from infection with L. prolificans was 46.9% in one series; however, the study included immunocompetent patients with localized disease; the case-fatality rate was 87.5% in those with disseminated disease.
Clinical manifestations
The clinical manifestations of invasive fusariosis and scedosporiasis are similar in many respects to those of invasive aspergillosis, as described in detail in Chapter 24 , with distinctive features outlined in the following text.
Fusariosis.
The most common sites of invasive fusariosis in immunocompromised persons are the skin (60% to 80% of cases), lungs (50% to 80% of cases), and sinuses (20% to 30% of cases). , Fusarium can develop yeastlike adventitious sporulation within infected tissue, which facilitates dissemination, seen in 70% of cases. Unlike other molds that infrequently cause detectable fungemia and are difficult to recover in standard blood culture media, blood culture results are positive for Fusarium in 40% to 50% of cases.
Radiographic series comparing findings of pulmonary fusariosis with those of invasive aspergillosis and mucormycosis note that the halo sign (a nodule surrounded by ground-glass opacity) is frequently absent in cases of fusariosis. However, children with IMDs of all types often lack characteristic radiographic features such as the halo sign, so this distinction may not be applicable to younger patients.
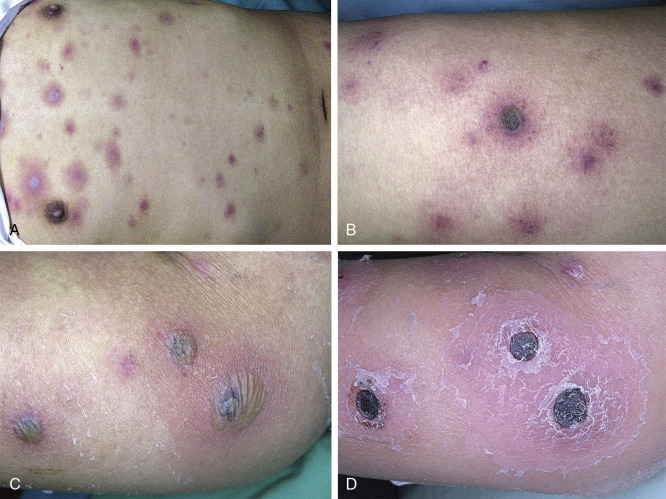
Cutaneous lesions of invasive fusariosis ( Fig. 25.4 ) are distinctive, consisting of painful, circular macules or papules, usually with central necrosis and surrounding erythema, similar in appearance to ecthyma gangrenosum. The appearance of cutaneous lesions in invasive fusariosis is usually secondary to hematogenous dissemination to the skin, rather than direct inoculation into the skin. A solitary lesion may develop initially, usually with progression to multiple lesions, mostly distributed on the extremities.