Molecular Biology of Prostate Cancer
Felix Y. Feng
Arul M. Chinnaiyan
Edwin M. Posadas
INTRODUCTION
Prostate cancer is the most common malignancy and the second leading cause of cancer death in men in the United States.1 It also poses a global problem, with recent significant increases in incidence outside the Western world.2 This malignancy is marked by extremely clinical and biological heterogeneity, which creates the opportunity for resistant clones to emerge, leading to eventual relapse and disease progression, and thus presenting problems for both patients and their physicians.
A major limitation in the management of this disease has been in the nomenclature used to describe the nature of each patient’s underlying disease. The currently employed staging system and the histopathologic scores established by Gleason reflect neither our current understanding of prostate cancer biology, nor the implications of this biology for outcomes and management. There has remained a need to further refine the pathologic classification of prostate cancers to account for the various families of molecular aberrations that exist within this disease. It is hoped that this refinement of classification may allow for more precise or “personalized” management of prostatic adenocarcinomas.
Systemic therapy for this disease begins with the reduction of circulating testosterone levels by medical or surgical castration. In most cases, this results in clinical benefit. However, progression to castration-resistant prostate cancer (CRPC) eventually occurs. A number of effective CRPC therapies have recently emerged. Regretfully, none are curative and all have a limited benefit, as shown in clinical trials. Refining our understanding of the molecular nature of this disease will hopefully lead to better characterization and, ultimately, more effective assignment of treatments to improve outcomes for patients.
OVERVIEW OF THE GENOMIC LANDSCAPE OF PROSTATE CANCER
The advent of next-generation sequencing technology has significantly broadened our understanding of the genomic landscape of prostate cancer. Recent studies have characterized the complete prostate cancer genome from 75 patients and have reported on the exomes of hundreds of additional cases.3,4,5,6,7 In combination with gene expression and copy number data previously interrogated from thousands of tumors, these studies provide a relatively comprehensive profile of the prostate cancer genome and transcriptome. Like other epithelial tumors, prostate cancers harbor genomic lesions, such as amplifications and deletions; point mutations; and translocations, as well as transcriptional changes leading to overexpression of oncogenes and underexpression of tumor suppressor genes.
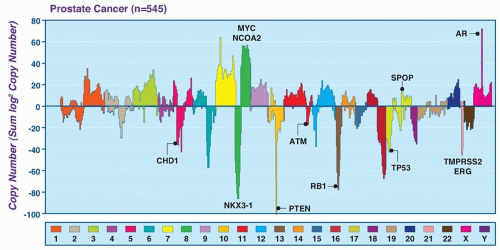
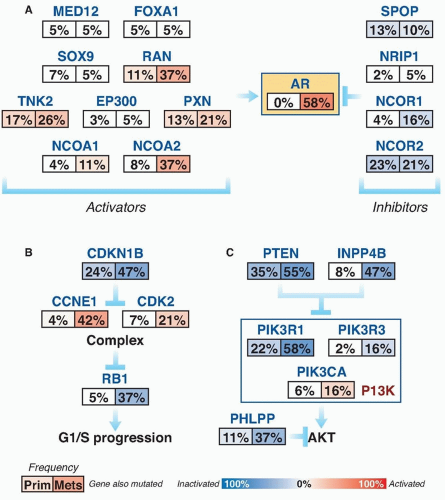
Subsets of tumors have been shown to harbor recurrent amplifications and deletions ranging from focal alternations (one to a few genes) to others that span entire chromosomal arms (Fig. 41.1). These alterations tend to increase in prevalence with higher grade and stage. The most common deletions occur on chromosomes 8p, 10q, and 13q, and include genes such as NKX3-1, PTEN, and RB1. Metastatic tumors harbor amplifications of chromosome X and 8q, which include the androgen receptor (AR) and MYC oncogenes (see Fig. 41.1). Although the prostate cancer genome exhibits an overall low mutation rate (˜1 per MB), the landscape of prostate cancer can also be defined by copy number and mutational alterations along several key oncogenic and tumor suppressor pathways, which are commonly involved when the individual genes in each pathway are considered collectively. Three of these pathways—the AR pathway, the phosphatidylinositol 3-phosphate kinase (PI3K)/AKT pathway, and the retinoblastoma (RB) pathway—are altered in more than one-third of primary cancers and in the vast majority of metastatic lesions.3,4,5,6,7 Frequent alterations in these pathways suggest that, in prostate cancer, different individual genes in the pathway may be targeted to activate or suppress a common pathway lesion (Fig. 41.2).
In addition to pathway-based analyses, prostate cancer can be defined by recurrent lesions of single genes. Approximately 50% of prostate-specific antigen (PSA)-screened prostate cancers harbor gene fusions involving E26 transformation specific (ETS) transcription factors, which represent oncogenic drivers.8,9 Strikingly, ETS fusions and certain other lesions, such as mutation of the E3 ubiquitin ligase SPOP and fusions involving RAF, appear to be mutually exclusive, suggesting a framework for molecular subtyping of prostate cancer.3,10,11 The sections that follow further describe the altered pathways and recurrent genetic lesions most validated in prostate cancer.
ANDROGEN RECEPTOR BIOLOGY AND THERAPY
Introduction to the Androgen Receptor
AR has remained one of the most important and most studied proteins in prostate cancer. The dependence of most prostate cancers on AR activation has served as a basis for important therapeutic approaches, such as luteinizing hormone releasing hormone (LHRH) analogs, antiandrogens, and androgen biosynthesis inhibitors.
AR is a 110-kb steroid receptor transcription factor, located on Xq12.12 Upon binding to an androgen, AR mediates the transcription of a number of genes involved in survival and differentiation of prostate epithelial cells, starting at development of the normal gland. Expression of AR is high in the luminal epithelial cells but lower in the basal epithelial cells that define glandular structure.13
In conjunction with NKX3.1 and FOXA1, AR plays a critical role in normal prostate organogenesis and disease progression.14 NKX3.1 is an AR-regulated prostatic tumor suppressor gene that is located on chromosome 8p and present through development.15 It is the earliest marker of organogenesis and is involved with ductal morphogenesis and secretory function.16 Mutations are infrequent in human cancers, but loss of heterozygosity in 8p becomes more frequent during cancer progression, with 86% loss in prostate cancers (see Fig. 41.1).17 Loss of NKX3.1 has been associated with
activation of the TMPRSS2-ERG gene fusion, which promotes tumorigenesis.18 FOXA1 is a member of the forkhead transcription factor family that opens compacted chromatin to facilitate AR recruitement.19 It is known to play a pivotal role in AR and steroid receptor function.20 It is now recognized that mutation and overexpression of FOXA1 can promote progression to CRPC (see Fig. 41.2).3,5
activation of the TMPRSS2-ERG gene fusion, which promotes tumorigenesis.18 FOXA1 is a member of the forkhead transcription factor family that opens compacted chromatin to facilitate AR recruitement.19 It is known to play a pivotal role in AR and steroid receptor function.20 It is now recognized that mutation and overexpression of FOXA1 can promote progression to CRPC (see Fig. 41.2).3,5
Polymorphisms of AR as well as CYP17 and SRD5A2 are low penetrant risk factors for prostate cancer development in some but not all studies.21 AR-pathway alterations exist in 60% of primary tumors and nearly 100% of metastatic tumors. NCOA2 (also called SRC2) is the most commonly altered member of this pathway (see Fig. 41.2) and is an AR coactivator that potentiates transcriptional output. Several other AR coactivators, including NCOA1, TNK2, and EP300, are upregulated in metastatic disease, whereas AR corepressors, including NRIP1, NCOR1, and NCOR2, are downregulated (see Fig. 41.2).
Targeting Androgen Receptor Activity: GnRH-Targeting Therapies and Androgen Biosynthesis Inhibitors
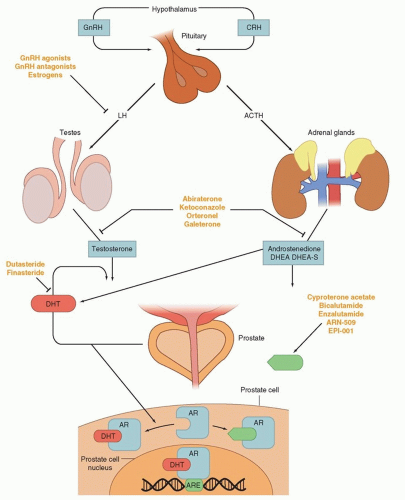
The inhibition of androgen synthesis has been used a means of suppressing AR activity. Gonadotropin-releasing hormone-luteinizing hormone (GnRH) agonists or antagonists represent the first-line approach for inhibiting androgen synthesis (Fig. 41.3). These therapies work by suppressing the hypothalamic-pituitary-gonadal axis by eventually causing luteinizing hormone (LH) levels to fall, thereby minimizing testicular production of testosterone and its subsequent conversion to dihydrotestosterone (DHT) by 5-alpha reduction. DHT is the most potent activator of AR and binds to the AR, resulting in translocation to the nucleus and activation of transcription (see Fig. 41.3).
These strategies, although initially highly effective, are often overcome during tumor progression. Alternative sources of DHT and alternative means of AR activity have been identified as mechanisms of resistance. GnRH-targeting agents have no impact on nongonadal androgen synthesis (i.e., suppression of adrenocorticotropic hormone [ACTH] and, hence, adrenal androgen synthesis) (see Fig. 41.3). The adrenal glands can synthesize adequate levels of androgens to promote cancer growth. CYP17 is a key P450 enzyme in the androgen biosynthesis pathway that generates dehydroepiandrosterone (DHEA) and androstenedione in the adrenal glands. These weak androgens can be further converted into testosterone or alternative steroid substrates that are reduced to DHT.22,12 This step is recognized as a particular bottleneck in androgen production. The specific inhibition of CYP17 decreases androgen synthesis, with less effect on the production of other essential steroids. Abiraterone acetate is a pregnenolone derivative that is a high-affinity, irreversible inhibitor of CYP17, and results in decreased adrenal androgen synthesis. At higher doses, this agent inhibits other DHT synthetic pathways such as 3β-hydroxysteroid dehydrogenase.23
Intratumoral androgens may be increased in CRPC.24 Measurements of intratumoral androgens showed that CRPC tumors have more testosterone than primary tumors in untreated men.25 Expression profiling studies comparing CRPC with primary tumors have shown that androgen synthesis enzymes are upregulated in CRPC.26,27 Patient samples at all stages of disease contain an abundance of AKR1C3 and SRD5A1, which are necessary for the conversion of androstenedione to DHT. However, the samples lacked high expression of enzymes necessary for de novo steroidogenesis.28 These data suggest that autocrine androgen synthesis may allow tumors to grow despite low serum androgen levels, but that this process may, in part, be dependent on adrenal precursors.
Targeting Androgen Receptor Activity: Antiandrogens
Antiandrogens compete with endogenous androgens for the ligand binding pocket of AR (see Fig. 41.3). Older agents promote translocation of AR from the cytoplasm into the nucleus and DNA binding, but induce conformational changes that prevent optimal transcriptional activity. In the United States, there have been
three historic nonsteroidal antiandrogens (NSAA): flutamide, bicalutamide, and nilutamide. Each has been associated with an antiandrogen withdrawal effect, whereby treated patients experiencing disease progression on treatment derive clinical benefit when the antiandrogen is stopped.29 Although this was initially believed to be related to AR mutation, later studies have shown this is due to AR activation by these NSAAs.30,31
three historic nonsteroidal antiandrogens (NSAA): flutamide, bicalutamide, and nilutamide. Each has been associated with an antiandrogen withdrawal effect, whereby treated patients experiencing disease progression on treatment derive clinical benefit when the antiandrogen is stopped.29 Although this was initially believed to be related to AR mutation, later studies have shown this is due to AR activation by these NSAAs.30,31
Newer NSAAs have now been developed that lack agonist effects on the AR. Enzalutamide (MDV3100) was approved by the U.S. Food and Drug Administration (FDA) in 2012.32 Other next-generation NSAAs are in development, including ARN-509.
Finally, new approaches are being taken to inhibit AR at the amino terminus.33 As opposed to interrupting ligand binding, agents such as EPI-001 interfere with transcriptional activity of AR. These agents are still in early development, but appear very promising because they display activity even when the ligand-binding domain of AR has been altered.
Alterations in the Androgen Receptor During Prostate Cancer Progression
Prostate cancers eventually progress to a more lethal state: castration resistance. The time to development of resistance and the molecular pathways to CRPC differ from patient to patient. Progression of CRPC is usually accompanied by the restoration of PSA secretion, indicating a reactivation of AR activity in these cases.12
Gene amplification and increased protein expression represent the most common mechanisms of means of reactivating AR signaling in the face of castration therapy. These changes allow cancer cells to respond to subphysiologic concentrations of androgen.34 AR mutations are detected in up to 10% of CRPC cases and allow for activation by alternative ligands.35 Overexpression of AR is seen in approximately 30% of cases of CRPC.36 Alternative splicing of AR mRNA can lead to a truncated protein missing the ligand-binding domain. These increase in number in the face of many second-line NSAAs.37,38 Some of these variants have constitutive transcriptional activity in the absence of androgens. Overexpression of these variants can confer castration resistance in preclinical models.39 More recent studies show that these variants impact sensitivity to taxanes40 and even next-generation antiandrogens such as enzalutamide.41
ADDITIONAL PATHWAYS FREQUENTLY ALTERED DURING DISEASE PROGRESSION
The Phosphatidylinositol 3-Phosphate Kinase (PI3K) Pathway
Progression to CRPC is characterized by gain of function in AR and activation of the phosphatidylinositol 3-phosphate kinase
(PI3K) pathway. Cross-talk between these pathways has been proposed42,43 because a loss of PTEN and subsequent PI3K activation is associated with repression of androgen responsive genes. Conversely, AR inhibition results in the upregulation of PI3K activity.42,43 As such, combination therapy with PI3K and AR inhibition are currently being evaluated in clinical trials.
(PI3K) pathway. Cross-talk between these pathways has been proposed42,43 because a loss of PTEN and subsequent PI3K activation is associated with repression of androgen responsive genes. Conversely, AR inhibition results in the upregulation of PI3K activity.42,43 As such, combination therapy with PI3K and AR inhibition are currently being evaluated in clinical trials.
The PI3K signaling cascade is one of the most commonly altered pathways in human malignancy. PTEN is a tumor suppressor that deactivates PI3K signaling. PTEN deletions are present in nearly 30% of primary prostate cancers, and inactivating mutations of PTEN occur in another 5% to 10%; both are more common in advanced disease (see Fig 41.2).3,5,7,44,45 Functional studies across multiple preclinical model systems of prostate cancer consistently reinforce the role of PTEN in suppressing tumorigenesis and prostate cancer progression.46,47 Dysregulation of PTEN represents a poor prognostic factor. There is significant evidence that PTEN deletion is associated with higher stage, higher Gleason grade, and higher rates of progression, recurrence after therapy, and disease-specific mortality.48,49
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree