Mltidisciplinary Reconstruction of the Head and Neck: General Principles
Mark L Urken
Adam S. Jacobson
Daniel Buchbinder
Devin J. Okay
Cathy L Lazarus
As the management of head and neck cancer has evolved over the last decade, so too has the reconstruction of defects created by ablative surgery. Because of the level of confidence in transferring tissue of various types, thickness, and dimensions to achieve the desired goal, the overall level of sophistication has increased in the various subsites that are restored. As head and neck reconstructive techniques have evolved from the era of pedicled flaps to that of free tissue transfers, the focus has become less of simply covering a defect or “closing a hole” but rather addressing the multifactorial problems of restoring a region to a level of form and function that best replicates the predisease state.
The last two decades have been dominated by the application of microvascular surgery to the head and neck; the early emphasis was on discovering new donor sites for hard and soft tissue and later on a more detailed understanding of the recipient site required to select the best reconstructive technique. Just as head and neck cancer demands a multidisciplinary approach that tailors the treatment of the disease, so too does the reconstructive component in which there is a need for several disciplines working in a collaborative manner. The reconstructive/rehabilitative team should include a reconstructive surgeon, an oral and maxillofacial surgeon, an oral and maxillofacial prosthodontist, an oculoplastic surgeon, and a speech pathologist. Preoperative assessment of each patient by the appropriate disciplines helps ensure that all considerations are taken into account before embarking on the implementation of the reconstructive plan. A careful assessment of the tumor and a prediction of the anticipated three-dimensional defect will help the team anticipate the need for hard and soft tissue as well as prosthodontic rehabilitation. The reconstructive team must determine the goal for each individual patient, which is a balance determined by the components of the defect, the biology of the disease, the comorbidities and motivation of the patient, the wound-healing properties of the recipient site determined by exposure to prior therapy, the patient’s nutritional status, and their continued usage of alcohol and tobacco. The goals, therefore, may vary for different patients faced with the same subsite defect because of these variables. If bone is to be a part of the reconstruction, then determination of the method of fixation must be established to ensure flap stability until bony union has occurred. In pediatric patients, there are special considerations related to the impact of bone harvest on the donor site. In addition, there is often a preoperative plan to remove fixation hardware in the early postoperative period, so that growth of the maxillofacial skeleton can continue unencumbered by rigid framework. Meticulous execution of the reconstructive plan must be followed by the appropriate rehabilitative therapy to maximize the recovery of function. The reconstructive team must be dynamic in its approach so that unforeseen circumstances that arise can be addressed during surgery. A positive surgical margin, for example, can substantially alter the reconstructive plan; a larger flap or even one from a different donor site may be required to accomplish the desired goal. Outcome measures specific to each subsite should be critically evaluated to determine the level of functional recovery. The monitoring and evaluation of function that results from reconstructive efforts are essential to the continued progress of these sophisticated and essential techniques. The continued refinement of approaches and the determination of the optimal reconstructive strategies depend on it. Centers vary their approach based on the collective experience and professional expertise within their respective institution, ideally based on this process of critical assessment of the outcome of their reconstructive approach.
The classification of defects is a critical undertaking that allows the reconstructive team to organize its approach to a particular subsite and to communicate, predict, and evaluate functional outcomes. Just as cancer staging provides a common lexicon for treating physicians that leads to therapy selection, prognostication, therapeutic outcomes, and to grouping of similar patients for outcomes analysis, defect classification does the same in head and neck reconstruction. The staging systems that are used to define cancers in particular subsites of the head and neck do not allow sufficient definition of the defect to permit accurate treatment planning. The size of an oral cancer and whether it has invaded mandible or maxilla determines the T stage for that cancer, but it does not provide the necessary ingredients for an accurate selection of the optimal reconstructive technique. This is determined by predicting what soft tissues and how much bone is to be removed. By estimating this, a more meaningful determination of what tissues must be harvested to restore form and function. Cancers of similar dimensions arising in the tongue and the buccal mucosa may lead to similar oncologic staging, but create very different demands for the reconstructive endeavor. Similarly, invasion of bone of the hard palate and the mandible may lead to a comparable T4 oncologic stage, but very different needs and strategies for functional reconstruction/rehabilitation. Moreover, different portions of the mandible and the presence of dentition will lead to very different reconstructive considerations that must be accounted for in the classification of that defect, none of which is addressed in the tumor staging.
The desirability of using prosthetic restoration, whether it be for dental, auricular, nasal, ocular, or facial restoration, must be determined in the early stages of treatment planning and must be communicated to the patient in a very thoughtful discussion in which they are allowed to articulate their concerns regarding the use of prosthetics versus native tissue to achieve the final goal. Although the restoration of dentition with a conventional denture or more favorably with an implant-supported prosthesis is very well accepted, patients, more often than not, have very strong feelings regarding the application of a prosthetic solution, instead of their own tissues, for restoration of a part or all of the ear, nose, or face. In the past, the concern about loss of retention of the prosthesis to the facial tissues often led to less favorable patient satisfaction. This has largely been overcome by the application of osseointegration and fixation to achieve prosthetic stability and retention. However, there remains a significant bias by patients regarding the use of a prosthetic nose or ear that may lead them to select reconstruction, albeit with the acceptance of a less favorable aesthetic result. It is imperative that the reconstructive team embarks on very frank discussions with the patient and his or her family regarding the availability of a skilled prosthodontist and a skilled reconstructive surgeon with experience in restoring the particular defect at issue. Patients with devastating defects must be provided realistic counsel and perhaps offered alternative centers to undergo treatment. If implants are to be placed to achieve the desired outcome, then consideration must be given to the volume and quality of the underlying bone, the quality of the overlying soft tissue, and the timing of implant placement with respect to the institution of adjuvant radiation. Radiation alters the process of osseointegration and, therefore, compromises the overall success of the implants. Discussion of implant placement and radiation will be provided later in the chapter.
Although much of the emphasis in contemporary head and reconstruction surgery is placed on achieving an optimal restoration, the ability to shorten the recovery from ablative/reconstructive surgery has significant implications for the outcome of the cancer treatment. It is therefore essential for the reconstructive team to outline a restorative strategy that achieves a suitable “packaging” of the entire surgical treatment and recovery in an appropriate time period. The achievement of these goals depends, of course, on the performance of these reconstructive efforts by experienced teams of experts who are able to accomplish this in a predictable and reproducible manner.
The debate of whether it is more appropriate to perform a delayed or primary reconstruction has been waged over the past several decades as primary reconstruction has emerged as a highly predictable and reliable technique. The notion that a delay in reconstruction helps to make patients appreciate more fully the delayed reconstructive efforts may be valid in select subsites such as the nose and the auricle. More often than not, however, such an approach leads to inferior reconstructive results for mandible and maxilla, where primary reconstruction offers superior outcomes. The use of surveillance imaging performed in a sequential manner during the posttreatment period provides a useful method of monitoring the reconstructive site once the bone has been placed to restore the defect. Granted, the hardware that is used to achieve rigid fixation somewhat distorts, and therefore compromises the images, but despite these associated problems the value of improved function and dental rehabilitation that can be achieved by primary reconstruction far outweighs the drawbacks. Specific functional outcome studies for each of these two subsites will be discussed in detail later in the chapter.
In addition to the variables specific to the patient, the selection of donor sites for both hard and soft tissues will be influenced greatly by the experience level of the reconstructive team. The adage that “simple is better” continues to have relevance in reconstructive decision making, that is to say, the concept of starting with simple and moving to complex methods should be considered for each reconstructive challenge. Although selection of a simpler technique may utilize a less demanding procedure, it is imperative that all aspects of that procedure be anticipated and understood. A good example of that planning discipline is the decision-making process for reconstructing the floor of mouth. Although it may be tempting to use a split-thickness skin graft for the sake of expediency, the surgeon and the patient must understand the implications of failure of “graft take” on the mobility of the tongue and the architecture of the oral cavity. It must be understood that if an untoward outcome such as loss of a graft should occur, then wound contracture will result in a compromised functional result that can only be partially corrected by further reconstructive efforts.
There are a number of regional flaps that continue to play an important role in the management of patients with head and neck cancer. Certain donor sites, such as the forehead were commonly used as a source of flaps for head and neck reconstruction in the 1960s, but are rarely utilized in contemporary reconstruction. There does, however, remain an important role for the pectoralis major flap, the deltopectoral flap, the trapezius flap(s), and the latissimus dorsi flap. Additionally, there are newer regional sources of soft tissue such as the submental flap, the temporalis and temporoparietal flaps, and the posterior scalping flap. The role of regional flaps serving as carriers for vascularized bone has largely been eliminated due to the emergence of flaps harvested from the fibula, the scapula, the iliac bone, and the radius, which are all much more reliable donor sites for the restoration of the maxillomandibular skeleton.
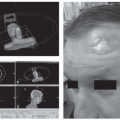
In the contemporary era of head and neck cancer management, there are certain subsites such as the oropharynx and the laryngopharynx in which ablative surgery is usually reserved for the salvage setting or for less common types of neoplasms such as those arising from the minor salivary glands. There are centers where primary surgery, whether endoscopic, robotic, or open, is applied. It is safe to say, however, that for both the oropharynx and the laryngopharynx, the role of surgery is usually relegated to failures of primary radiation with or without chemotherapy. There are special considerations when performing reconstruction in a patient who has severely compromised tissues as a result of extensive prior treatment. An increased likelihood of wound breakdown and salivary fistula must be accounted for in the reconstructive strategy, and every effort to prevent these sequelae should be instituted. This usually includes the importation of healthy muscle to provide coverage of the carotid artery as well as the microvascular pedicle if a free flap has been transferred; this muscle may come with the free-flap donor site or may require a second donor site, often the pectoralis major muscle flap (Fig. 8-1). There are additional issues related to the availability of recipient vessels in patients who have undergone extensive prior surgery that often makes the conventional recipient vessels unsuitable for use in a microvascular transfer. Knowledge and experience in harvesting recipient vessels, such as the thoracoacromial vessels or the internal mammary artery and vein, are sometimes required to achieve a successful reconstructive result.1,2
In keeping with the organization of much of this book, this chapter has been organized according to the various subsites of the head and neck. In each subsite discussion, the major considerations for reconstruction and the defect classification scheme that the authors feel best defines the extent of the reconstructive problem will be presented. In addition, functional outcomes studies, when available, will be reviewed to help understand the impact of the reconstructive techniques on the functional recovery of the patient. A section covering various topics in maxillofacial prosthodontics will be presented to complete a comprehensive
discussion of contemporary head and neck reconstruction/rehabilitation. Unfortunately, the constraints on the length of this chapter do not permit every subsite to be presented. The authors have selected the most common subsites and the topics in which new data are available since the publication of the prior edition of this text.
discussion of contemporary head and neck reconstruction/rehabilitation. Unfortunately, the constraints on the length of this chapter do not permit every subsite to be presented. The authors have selected the most common subsites and the topics in which new data are available since the publication of the prior edition of this text.
 FIGURE 8-1. Types of regional and free microvascular, osteocutaneous, and enteric flaps. (See color insert.) |
ORAL CAVITY RECONSTRUCTION
Tongue
From the perspective of oral function, the tongue is undoubtedly the most important structure within the oral cavity and its substance and mobility should be preserved whenever possible. Under all but the most extraordinary circumstances, the tongue should not be utilized as a source of mucosa for reconstruction of defects of the oral cavity. In contemporary head and neck cancer management, the oral cavity and therefore cancers of the mobile tongue, the floor of mouth, the gingiva, the palate, and the buccal mucosa are most frequently managed by primary surgery. Although in most centers the treatment of base of tongue squamous cell cancers is by radiation, with or without chemotherapy, there are circumstances in which salvage surgery and treatment of nonsquamous cancers requires the resection and reconstruction of the tongue base. Usually the latter resections include portions of the oropharynx and occasionally the supraglottic larynx. Extirpation of mobile tongue and floor of mouth cancers can be done very effectively and in a manner that preserves oral function. The decision as to whether the mandible should be included in the resection, in the form of a marginal or segmental resection, is discussed in detail in Chapter 16.
Classification of Glossectomy Defects
Several years ago, the authors’ team established a defect classification scheme for glossectomy defects that was designed to assist with the communication between clinicians, to assist with treatment planning, and to allow clinicians to determine the prognosis for functional recovery based on the extent of the tongue removed and the integrity of the innervation to the remaining tongue.3 At that time, the tongue was divided into mobile and base of tongue defects and further subdivided into quarters to help quantify the extent of the resection. The status of
the motor (hypoglossal) and the sensory (lingual) nerve supply to the tongue was also included in that scheme.
the motor (hypoglossal) and the sensory (lingual) nerve supply to the tongue was also included in that scheme.
One of the premises of any reconstructive scheme is that the subdivisions are reflective of the contemporary techniques that are available for reconstruction. Toward that end, the authors have since modified the classification scheme to better reflect the reconstructive approach currently in use.
The separation of the tongue base and the mobile tongue has been retained, and the authors have further subdivided the mobile tongue into the following defects: minimal glossectomy defects that do not involve the floor of mouth, minimal glossectomy defects that do involve the floor of mouth, subtotal glossectomy defects that preserve a portion of the mobile tongue that has intact muscle and an intact nerve supply, and total mobile tongue defects. The total mobile tongue defect is further subdivided into those with an intact mandible and defects that are associated with a segmental mandibulectomy. The base of tongue is divided into three categories of minimal glossectomy, subtotal glossectomy, and total base of tongue resection. Finally the total glossectomy defect is subdivided between those that have an intact mandible and those that are combined with a segmental mandibulectomy.4
Goals of Reconstruction of the Tongue
The fundamental goals of reconstruction of the tongue are to restore the patient’s ability to swallow without aspiration and to form words with the greatest possible clarity. Conversational speech both in person and by telephone can be accomplished with a level of effectiveness that permits the patient to resume his or her premorbid quality of life (QOL). There are important components that assist toward that end. Retention of tongue mobility is critical to success and when there is a portion of the tongue that retains a motor supply, then the goal is to restore the missing part with redundant tissue so as to prevent tethering of the tongue to the inner table of the mandible or to the floor of mouth. Restoration of sensation is also an important goal in surgery and assists in giving the patient valuable feedback as to the location of food particles in the oral cavity. With this capability, the patient is better able to move those particles between the occlusal plane for mastication and propel them posteriorly to initiate the pharyngeal phase of deglutition. One of the key goals of restoring larger glossectomy defects is to match the neotongue to the volume of the oral cavity and the pharyngeal chamber. Therefore, any loss of movement can be compensated for by the restoration of volume that preserves the ability of the tongue to contact the palate and the walls of the pharynx, respectively.
Reconstructive Options
Restoration of mobile tongue defects depends greatly on the amount of the tongue that is resected and whether the floor of mouth mucosa has been preserved. The major consideration noted earlier is related to the preservation of movement and the restoration of volume, which becomes a more significant concern when the size of the defect increases and when the anticipated loss of mobility of the tongue is greater. For minimal glossectomy defects that do not involve the floor of mouth, primary closure provides a simple and effective means of restoring function. However, for minimal glossectomy defects that include the floor of mouth, there is a need to introduce thin pliable tissue to prevent tethering that would otherwise occur with primary closure to the gingival tissue. The workhorse reconstruction for that purpose is the radial forearm free flap that usually provides the thinnest available tissue and which provides excellent lining for the oral cavity (Fig. 8-1). This donor site has a well-defined sensory distribution and large sensory nerves (the medial and the lateral antebrachial cutaneous nerves) that are easily incorporated in the harvest of tissue from the volar surface of the forearm.5
As mobile tongue defects increase in size, the need for more tissue is balanced by the integrity of the motor nerve supply and the amount of muscle in the tongue remnant. Through the incorporation of de-epithelialized segments of the forearm soft tissue, bulk can be added to the reconstruction by placing that vascularized subcutaneous tissue under the skin which is used to reline the tongue and the floor of mouth.3 Alternative donor sites such as the lateral arm flap and the anterolateral thigh (ALT) flap (Fig. 8-1) provide an opportunity to transfer skin with more subcutaneous tissue than is usually present in the radial forearm donor site.
After resection of the total mobile tongue and a segment of the mandible, the reconstruction of the oral cavity requires two separate but integrated strategies for replacing the bone and the soft tissue. In this type of reconstruction, the goal is to match the volume of the neotongue with the volume of the oral cavity. Alternatively, a palatal prosthesis can be used but that involves coverage of the intact palate and the loss of a valuable source of sensate mucosa. One approach that the authors have adopted is the transfer of an iliac crest free flap (Fig. 8-1) with the associated skin paddle and placing that bone in a horizontal orientation so that it serves as a platform for support of the soft tissue. Although this is opposite the usual orientation of the iliac bone (placing it vertically for restoration of the mandibular alveolus), the technique provides valuable support of the neotongue reconstruction with a rigid platform that helps to prevent caudal migration of the soft tissue. Therefore, long-term contact between the neotongue and the palate is achieved.6
For total mobile tongue resections with an intact mandible, the technique that the authors employ is to transfer a large soft tissue flap that is inset in a manner that places the soft tissue above the occlusal plane. Achieving long-term stability of that reconstruction, so that gravity does not lead to a decrease in the height of the neotongue, requires a number of intraoperative maneuvers that simulate the strategy noted earlier whereby the goal is to maintain contact of this static reconstruction with the palate. One such maneuver was noted earlier where the introduction of supplemental soft tissue under the skin provides added bulk that is preserved due to the fact that it is vascularized tissue. In addition, suturing the supplemental tissue to drill holes in the lower border of the mandible helps to form a platform to support the overlying neotongue. The authors described the use of a rectus abdominus flap (Fig. 8-1) where the anterior rectus sheath is sutured to the lower border of the mandible and thereby all of the soft tissue above that fascial layer is supported. The muscle component that the authors anticipate will atrophy is below the fascia and does not contribute to the anticipated bulk.6
The reconstruction of the base of tongue is very similar to that of the mobile tongue with respect to the goal of achieving primary closure when possible and the introduction of increased bulk to match the volume of the pharyngeal chamber. In the salvage setting, the performance of surgery in this region involves more liberal indications for introducing vascularized tissue to aid the wound-healing process that usually has been substantially compromised by radiation therapy. These defects often involve adjacent anatomic structures, such as the pharyngeal walls and the soft palate (SP), all of which must be addressed at the same time. Primary closure of smaller pharyngeal defects with native tissue helps narrow the caliber of the chamber that places less of a demand on the base of tongue. The restoration of this region has greater functional implications for the patient with respect to safe and effective swallowing. The more efficient the swallowing, the less likely the patient is to aspirate, which when significant can lead to dependence on alternative routes of enteral alimentation such as a gastrostomy tube.
Although the restoration of a dynamic component of the tongue is a laudable goal, to achieve this in a way that actually provides functional neotongue movement that contributes to swallowing and articulation is very challenging. Movement alone, or demonstration of electromyographic (EMG) activity in transferred muscle that is reinnervated through the hypoglossal nerve (HG), does not constitute appropriate tongue reanimation. It is critical that comparative studies with patients having comparable tongue defects be functionally evaluated following static reconstructions, described earlier, and dynamic reconstructions involving the transfer of reinnervated muscle. To date, such studies have not been conducted.
As a means of helping to overcome the loss of activity of the hyomandibular muscle complex, the authors have found laryngeal suspension to be a very useful maneuver following base of tongue or total tongue resection. By statically positioning the larynx in a more cephalad and more anterior position, the larynx is placed in a more favorable functional position for avoiding and/or minimizing aspiration.
Functional Outcomes of Tongue Reconstruction
Very little meaningful outcomes research has been done comparing the results of various reconstructive techniques for the specific defects that are mentioned in this discussion. Owing to the lack of sufficient patient volume in any one institution, there are no comparative institutional studies that will help the surgeon decide between various reconstructive options.
These authors have been examining outcomes following tongue resection with and without reconstruction (lateral and anterior resections). To date, oral outcome variables of tongue strength, tongue and jaw range of motion (ROM), and saliva production have not been extensively assessed in this population, nor have they been correlated with performance status on speech and swallowing and patient-rated QOL. Results in a cohort of 28 patients have shown only moderate reductions in lingual strength (mean = 35 kPa) as compared to healthy individuals. Jaw ROM was within normal limits (mean = 40 mm) and tongue ROM was a mean of 82 % of normal (utilizing a converted scale). Oral outcome variables of tongue strength, tongue ROM, and degree of jaw opening were found to significantly correlate with all three functional domains of the Performance Status Scale (PSS),7,8 including Normalcy of Diet, Eating in Public, and Understandability of Speech.9 Improved functioning on these oral outcome variables were associated with a more normal diet, greater ability to eat in public and improved understandability of speech.9
When examining oral outcome variables with patient-rated QOL, tongue strength, jaw opening, and saliva weight were significantly correlated with QOL outcomes for swallowing on the EAT-10,10 the MD Anderson Dysphagia Inventory (MDADI),11 and speech (the Speech Handicap Index).12 Those patients scoring higher on tongue strength, jaw opening, and saliva weight found their QOL to be improved for both speech and swallowing.
Objective performance status on the PSS significantly correlated with patient-rated QOL on all three QOL scales, with improved QOL correlating with a more normal diet and higher Eating in Public scores (i.e., more likely to eat out in public). Interestingly, no significant differences were found by surgical reconstruction type (i.e., free flap vs. primary closure) for PSS score or QOL score, since the primary closure group demonstrated smaller surgical defects and one might anticipate better performance in this group. However, tongue strength scores were significantly higher for the primary closure group, likely due to a greater amount of native tongue muscle.
When examining the effects of postoperative radiotherapy ± chemotherapy across tumor site and reconstruction type, those patients who underwent surgery >12 months from time of assessment and who underwent radiotherapy ± chemotherapy demonstrated significantly lower tongue strength scores than those seen in the surgery-only group. This is not surprising, since tongue strength has been found to be reduced in patients having undergone chemoradiotherapy for oral and oropharyngeal tumors.13
Oral outcome variables described previously have also been found to correlate with performance status on PSS and patientrated QOL in patients having undergone microvascular free-flap reconstruction only, including the lateral tongue, the anterior tongue, and the total tongue sites.14 Tongue strength was highest in the lateral tongue resection group, as assessed with the Iowa Oral Performance Instrument (IOPI). This device measures tongue force, with placement of an air-filled bulb on the tongue and instruction to press upward with maximum pressure on the bulb against the palate.15 Three of five total glossectomy patients could perform a similar flap-resistance task and performed surprisingly well, demonstrating 46% of the mean strength seen in lateral tongue patients. No significant differences in Understandability of Speech were observed between groups. Total glossectomy patients scored 75% understandable speech, which was a better outcome than expected for this group of patients.
When examining overall QOL functioning in these three surgical groups utilizing the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire, Head and Neck Module (EORTC H&N35)16 significant correlations with the oral outcome variables were found for the Swallowing and Sticky Saliva domains, with greater jaw opening correlating with better Swallowing scores, and reduced jaw ROM and less saliva weight correlating with poorer Sticky Saliva scores.14 Patient-rated EORTC domains of Swallowing and Speech both correlated significantly with performance status domains of Normalcy of Diet and Understandability of Speech. Thus, patient-rated QOL correlated well with functional outcomes related to diet and speech.
It is clear that surgical resection and reconstruction affect functional outcomes after surgery. The amount of native tongue, degree of lingual mobility and strength, jaw ROM, and saliva weight after oral cancer surgery play a key role in speech and swallowing performance as well as overall QOL. As can be seen from these data, overall functioning across oral tumor site and reconstruction type can be quite high. In addition, patient perception of function correlates well with measured functional outcomes. Data on oral outcome measures in larger groups of patients should help predict functional outcomes and QOL after definitive resection and reconstruction for tongue cancer.
There are very little data concerning the role of sensation in producing a functional advantage for tongue reconstruction. However, Loewen and colleagues17 found similar sensation ability in patients who underwent oral cavity sensate flaps as compared to healthy individuals. Specifically, these authors found improvement in light touch and two-point discrimination on the tongue. They also found improvement in swallow functioning, with improved oral transit times and improved bolus clearance through the oral cavity, as well as increased diet options when comparing these patients to similar patients cited in the literature. The recovery of sensation after surgery remains somewhat controversial. Boyd et al.5 reported that some patients with nonsensate flaps placed into the oral cavity experience recovery of sensory feedback, presumably through the growth of sensory nerves from the perimeter and/or the depth of the wound into which the flap is being placed. This provides the most compelling information available on this topic.
In patients destined to undergo a substantial glossectomy, the surgeon must address fundamental questions relating to laryngeal function; specifically, whether he or she will be able to regain function with this organ in place or whether a functional laryngectomy should be performed at the time of primary
surgery to prevent aspiration. The prediction as to whether a patient will be able to swallow is often difficult and that uncertainty is heightened due to the nature of deglutition, which is multifactorial. When the disease mandates the removal of the larynx to obtain clear margins, the decision is easier. When the disease requires the removal of the supraglottic larynx, then protection of the airway is certainly compromised and the likelihood of safe deglutition is diminished. During the era when partial laryngeal surgery was performed as the mainstay of therapy, the effectiveness of the extended supraglottic laryngectomy in which a portion of the base of tongue was removed was well documented. However, the patient’s comorbid conditions, especially chronic lung disease and the amount of tongue that is to be removed certainly impact the likelihood of success. The irreversibility of a total laryngectomy usually leads us to avoid performing it in the primary setting, and often the procedure is reserved for the secondary setting after a period of adaptation and intensive therapy with an experienced speech pathologist. If the patient demonstrates an inability to protect the airway, then a functional laryngectomy can be performed in a secondary setting.
surgery to prevent aspiration. The prediction as to whether a patient will be able to swallow is often difficult and that uncertainty is heightened due to the nature of deglutition, which is multifactorial. When the disease mandates the removal of the larynx to obtain clear margins, the decision is easier. When the disease requires the removal of the supraglottic larynx, then protection of the airway is certainly compromised and the likelihood of safe deglutition is diminished. During the era when partial laryngeal surgery was performed as the mainstay of therapy, the effectiveness of the extended supraglottic laryngectomy in which a portion of the base of tongue was removed was well documented. However, the patient’s comorbid conditions, especially chronic lung disease and the amount of tongue that is to be removed certainly impact the likelihood of success. The irreversibility of a total laryngectomy usually leads us to avoid performing it in the primary setting, and often the procedure is reserved for the secondary setting after a period of adaptation and intensive therapy with an experienced speech pathologist. If the patient demonstrates an inability to protect the airway, then a functional laryngectomy can be performed in a secondary setting.
OROMANDIBULAR RECONSTRUCTION
Success in oromandibular reconstruction is no longer measured simply by achieving a bone restoration of the segmental mandibular defect, but rather by the ability to restore not only the form but also the function of the oral cavity. A great deal of effort has gone into the detailed three-dimensional analysis of composite defects and into selecting the best reconstructive option that will provide both the optimal soft and hard tissue to restore the given defect. In the past, the major emphasis was on the restoration of the bony defect, but with the highly predictable nature of microvascular transfer of composite flaps, the realization that functional recovery was largely dictated by the movement of the tongue, the emphasis shifted to the soft tissue restoration as well as the restoration of functional dentition. One of the most important considerations is the effect of the soft tissue flaps on the form and function of the tongue and the floor of mouth. After a partial glossectomy, the unpreventable sequelae are related to the loss of the intrinsic tongue musculature and the neurologic supply. Preservation or restoration of sulcular anatomy is important for oral competence, tongue mobility, and denture stability. Additional requirements in soft tissue reconstruction may involve the buccal mucosa, the SP, and the pharynx. Lastly, patients with composite defects involving bone, mucosa, and skin require external coverage as well.
Defect Classification System
A clear definition of the reconstructive options begins with a careful definition of the extent and nature of the defect using a practical classification that will direct the restorative effort. The shortcomings of the previously used classification schemes did not fully take into account the intricate, composite nature of the defects. Furthermore, the resulting neurologic deficit and its impact on the restoration of reasonable function was also largely ignored. The authors felt the need for a more encompassing, yet simple, classification system and in 1991 introduced a new oromandibular classification system to define the physiologic and aesthetic impact caused by the ablative procedure and the challenges that would be faced by the reconstructive surgeon in achieving the optimal functional restoration of the patient.3 It should be emphasized that although the structure of the mandible is not that difficult to duplicate, the removal of various portions of the mandible leads to disruption of various muscular attachments and, in addition, the components of the temporomandibular joint, both of which impact the function of the reconstructed lower jaw. This system was also designed to aid in the selection of the composite free tissue transfer that would provide the best combination of soft tissue and bone components to restore a given defect.
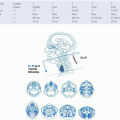
On the basis of the functional impact from resection of the mandibular condyle, lateral defects are divided into three zones: the condyle, ramus, and body (Fig. 8-2). The rationale for subdividing body and ramus defects is based on the functional impact that will result from the disturbance of the masticator muscle sling. Body defects are defined as the tooth-bearing segment that extends from an area just posterior to the canine tooth to the junction between the horizontal and vertical rami of the mandible (anterior to the attachment of the masticator muscle sling). Finally, ramus defects encompass the entire vertical ramus to the level of the subcondylar region. Symphyseal defects are more challenging to restore due in part to the shape of the bone but also the loss of support of the tongue that is produced when the suprahyoid and extrinsic tongue musculature are completely detached. Contouring of the bone flap to restore the symphyseal anatomy can be quite challenging, requiring multiple osteotomies which adds to the complexity of the reconstruction. Since the initial description of this classification scheme, the authors added the superscript M to denote a marginal resection of the mandible. The classification system also describes the associated soft tissue defects as mucosal defects of the oral cavity, defects of the tongue, and defects of the overlying skin.
Finally, the authors also felt the need to include both sensory and motor nerve deficits that result from the particular ablative procedure. These include deficits of the inferior alveolar, the lingual, the hypoglossal, and the facial nerves.
Goals of Oromandibular Reconstruction
With the major emphasis being placed on the quality of functional restoration, the following goals have been identified:
Reestablishment of mandibular continuity with a bonecontaining flap that will reasonably match the height and shape of the resected segment and will form a firm union at the interface with the native mandible
Reestablishment of the normal position of the condyles as well as normal occlusal relationships for prosthetic restoration and near-normal mandibular range of motion
Restoration of a functional perioral muscular sphincter to ensure oral competence
Restoration of sensation to the lips and oral soft tissues by the use of sensate flaps and nerve grafts when indicated
Preservation of tongue motion and restoration of tongue volume as outlined earlier
Restoration with a functional dental prosthesis.
Techniques of Oromandibular Reconstruction
The donor sites available for harvesting vascularized bonecontaining free flaps (VBFFs) have provided a menu of choices based on the particular qualities of the different soft tissue regions; therefore, a more critical selection of the ideal type of soft tissue for reconstruction is possible. When dealing with more complex defects, there is often no single flap that provides the ideal tissues to restore all of the missing parts. One of the key provisions is that an adequate amount of bone is supplied for the placement of endosteal fixtures to support a dental prosthesis, thereby restoring the occlusion. It is, however, the soft tissues of the flaps that will frequently determine the ultimate success of the reconstruction and hence soft tissue evaluation plays an important role in the selection of the donor flap.
Four donor sites are routinely used for oromandibular reconstruction: the fibula, the scapula, the iliac crest, and the forearm (Fig. 8-1). Each donor site has advantages and disadvantages related to the amount and quality of the bone and soft tissue needed, the caliber and length of the vascular pedicle, the donorsite morbidity, and, on a practical level, the necessary intraoperative positioning of the patient that will allow for a simultaneous two-team approach.
The fibula osteocutaneous free flap is supplied by the peroneal artery and venae commitantes. It provides bone of sufficient length (up to 25 cm) for reconstruction of subtotal segmental mandibular defects.18 The height of the fibula is significantly less than that of the normal mandible, making it more ideally suited for the reconstruction of the vertical ramus (including the condyle) as well as the mandibular arch in edentulous patients with a moderate degree of mandibular atrophy. When the defect lies within the tooth-bearing arch in dentate and edentulous nonatrophic patients, the height discrepancy between the reconstructed segment and native mandible must be addressed at the time of prosthetic restoration by the use of an implant supported mesostructure for a fixed denture prosthesis or an implant-assisted removable prosthesis. The skin paddle that can be harvested on the basis of septocutaneous perforators can be used to reline mucosal defects. There is usually a need to perform soft tissue debulking at the time of implant uncovering, owing to the thickness of the subcutaneous tissues and the fact that it is hair bearing. When used externally, the color match can be problematic. Finally, the distance of the donor site from the head and neck facilitates a simultaneous, two-team surgical approach.
The scapular system of flaps offers the widest array of soft tissue components, which accompany the 10 to 12 cm of bone that can be harvested from the lateral scapular border in an adult.19 The scapular and parascapular cutaneous flaps can be harvested, both of which are based on the circumflex scapular branches of the subscapular vessels. The angular branch of the subscapular vascular system supplies the tip of the scapula, allowing an independent vascularized segment of bone to be harvested. Inclusion of the latissimus dorsi and the serratus anterior muscles can also be accomplished and adds to the versatility of the composite flaps that can be harvested. The main advantages of this donor site are the number and mobility of the soft tissue components relative to the bone, which are supplied by independent vascular pedicles of the subscapular system. The disadvantages of this donor site relate to the variable bone stock that can be harvested from the scapula, which is often inadequate for the placement of dental implants, as well as the need for intraoperative repositioning of the patient into a lateral decubitus position, which makes a simultaneous two-team approach difficult, if not impossible.20
The iliac crest osteocutaneous flap is supplied by the deep circumflex iliac artery (DCIA) and vein, branches of the external iliac artery and vein.21 The vascular pedicle provides both an endosteal and periosteal supply to the iliac bone. The stock of bone that can be harvested is ideal for mandibular reconstruction, and the natural curvature of the ilium often assists in restoring the contour of the ipsilateral hemimandible.22 The skin overlying the crest is supplied by perforators that traverse the three muscle layers of the abdominal wall. One of the major limitations of this flap, when used in oromandibular reconstruction, is related to the skin paddle, which is often too bulky for use within the oral cavity. In addition, the skin has a relatively fixed relationship to the bone sometimes making it very difficult to restore the complex three-dimensional anatomy required to restore the composite defects. The addition of the internal oblique muscle based on the ascending branch of the DCIA greatly increases the versatility of this donor site.23 The thin pliable muscle, which undergoes atrophy after denervation, is ideally suited to restore gingival and mucosal defects over the neomandible. In such cases, the skin paddle is used for the restoration of cutaneous defects or simply as an external monitor of the circulation to the flap. A simultaneous two-team approach is possible. The major disadvantages of this donor site are the early gait disturbances and the potential for development of a ventral hernia. This can, to some extent, be avoided through meticulous closure of the donor site and the use of a mesh to augment the repair of the abdominal wall musculature.
The radial forearm free flap, which is based on the radial artery and the cephalic vein, provides thin sensate, pliable skin that can be transferred as an osteocutaneous flap. A segment of radius up to 8 to 10 cm and approximately 40% of its circumference can be transferred with the skin.24 The bone is of limited dimensions for structural and functional reconstruction of the mandible. The thin, pliable soft tissue component of this flap is ideal for use in relining the floor of mouth, the tongue, and the posterolateral pharyngeal wall defects. This makes the use of this flap ideal in a small subset of patients who have a very limited posterolateral segmental mandibular defects along with a significant soft tissue defect. In these patients, the missing mandibular segment is usually located beyond the dentate segment and, therefore, the inability to place implants is less important. The most significant complication associated with this flap is the overall 25% incidence of pathologic fracture of the remaining radius, an occurrence that can lead to a significant impairment in hand function.25 Fixation of the graft can also be problematic because it requires some stripping of the periosteum in the area of screw placement. This invariably leads to a decrease in the blood supply to the bone, which is periosteal in nature. These issues, coupled with the limited bone stock, has led to the infrequent use of this donor site for reconstruction of mandibular defects.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree