Microangiopathic disorders present with thrombocytopenia, hemolytic anemia, and multiorgan damage. In pregnancy, these disorders present a challenge both diagnostically and therapeutically, with widely overlapping clinical scenarios and disparate treatments. Although rare, a clear understanding of these diseases is important because devastating maternal and fetal outcomes may ensue if there is misdiagnosis and improper treatment. Microangiopathic disorders presenting in pregnancy are thus best assessed and treated by both obstetric and hematology teams. As a better understanding of the pathophysiology underlying each of the disease processes is gained, new diagnostic testing and therapies will be available, which will lead to improved outcomes.
Microangiopathic disorders in pregnancy present a challenge both diagnostically and therapeutically. Although these conditions are rare, they can lead to devastating maternal and fetal outcomes if misdiagnosed and treated improperly.
The microangiopathic disorders present with thrombocytopenia, hemolytic anemia, and multiorgan damage. The diagnosis of thrombotic thrombocytopenic purpura (TTP) has long been based on the clinical pentad of neurologic abnormalities, thrombocytopenia, microangiopathic hemolytic anemia, fever, and renal dysfunction. Hemolytic uremic syndrome (HUS) is often manifested by acute renal failure and hypertension, as well as microangiopathic anemia and thrombocytopenia. Renal dysfunction in HUS is often more severe than that in TTP, whereas neurologic abnormalities are more prevalent in TTP. HUS is more frequently observed in children who have had a preceding gastrointestinal infection; however, this condition has also been reported in adults and pregnant women. Patients with these diagnoses may or may not have hypertension, a hallmark of the disorders of preeclampsia.
Preeclampsia and hypertension, elevated liver enzymes, and low platelets (HELLP) are thought of as a spectrum of disorders, with HELLP being the most severe manifestation. Preeclampsia is defined as new onset of hypertension and proteinuria after 20 weeks’ gestation in its mildest form. Patients may progress and develop eclampsia, with neurologic abnormalities that are often attributed to the hypertension, such as headache, blurry vision, and seizures. Leaky cerebral vasculature also leads to cerebral edema, increased intracranial pressures, seizures, and coma. These severe neurologic symptoms may be seen at lower-than-expected pressures because of the widespread endothelial dysfunction. Women with severe preeclampsia may also go on to develop a hemolytic anemia and thrombocytopenia. HELLP, as the acronym implies, is diagnosed in patients who present with hypertension, elevated levels of transaminases, and thrombocytopenia. Patients may also have hemolytic anemia, disseminated intravascular coagulation, and other organ dysfunctions, even though liver dysfunction is often the predominant feature.
TTP and HUS are rare conditions in the general population, occurring in between 4 and 11 patients per million. TTP has a female to male predominance of 3:2. HUS typically occurs in children, and its incidence is approximately 0.64 of 100,000 in children younger than 15 years. Together, TTP-HUS occurs in 1 in 25,000 pregnancies. The association between TTP-HUS and pregnancy is in part due to the female predominance of the disease; however, pregnancy is thought to precipitate the disease as either a first occurrence or a recurrence in a patient with a history of TTP. In a review of published cases from 1964 to 2002, the frequency of pregnancy among women diagnosed with TTP-HUS was 13%.
Preeclampsia is much more common than TTP-HUS and affects approximately 5% to 8% of pregnancies. Whereas HELLP occurs in 0.5% to 0.9% of pregnancies, it affects 10% to 20% of cases affected by severe preeclampsia.
Pathophysiology
The hallmark of the microangiopathic disorders is the development of microthrombi in the small vasculature leading to thrombocytopenia, hemolysis, and specific multiorgan damage. Although TTP, HUS, and preeclampsia/HELLP syndrome are all microangiopathic disorders seen in pregnancy, the pathophysiologic mechanisms underlying each condition are different.
TTP
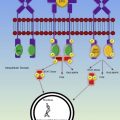
The development of microthrombi in TTP is now attributed to a deficiency in the von Willebrand factor (vWF) cleaving enzyme a disintegrin and metalloprotease thrombospondin type 1 repeats 13 (ADAMTS13). A deficiency of this metalloprotease, either inherited or acquired with autoantibody, leads to ultralarge vWF multimers that promote platelet aggregation within the microcirculation. The complex interaction of ADAMTS13 and vWF has been studied extensively, and shear stress is important for unfolding of the vWF protein, revealing the cleavage sites for ADAMTS13. This type of shear stress is found under normal conditions in arterioles and capillaries and promotes vWF-platelet interaction leading to aggregation and thrombus formation. The presence of ADAMTS13 is thus essential for the maintenance of normal hemostasis in the microvasculature. The severe thrombocytopenia that often occurs in TTP is a result of consumption in the microthrombi. Microangiopathic hemolytic anemia subsequently occurs due to mechanical injury within the thrombosed microvasculature.
Deficiency of ADAMTS13 may be either inherited or acquired. Numerous mutation types have been identified, including missense, nonsense, insertions, deletions, and splice-site variations. These mutations present with varying clinical severity, and approximately 90% of patients with either homozygous or compound heterozygous mutations have symptomatic TTP. For patients with no family history or known mutation, autoimmune inhibitors to ADAMTS13 are frequently observed. Of patients with a diagnosis of TTP, approximately 31% to 38% have a detectable inhibitor.
The causal relationship of pregnancy to the development or exacerbation of TTP is not fully understood. TTP is more frequent in women of childbearing age, perhaps this association is coincidental; however, some have argued that pregnancy can trigger these conditions. Although normal pregnancy is certainly a prothrombotic state with increases in levels of fibrinogen, vWF, and factor VIII (FVIII), there are also reductions in the level of ADAMTS13 to between 52% and 64% of the normal levels. For patients with a history of TTP and baseline lower levels of ADAMTS13, there is theoretically an increased propensity toward recurrence during this time. In a review of a case series in 2003, 14 women from 8 families with known familial TTP-HUS had their initial presentation during pregnancy. Whether pregnancy would prompt a relapse in a known patient with TTP or would induce inhibitor development in a patient with no prior history remains unclear.
HUS
Unlike TTP, HUS is thought to be associated with several abnormalities of the complement cascade, leading to the development of microthrombi, primarily in the renal parenchyma. The following 3 distinct types of HUS have now been described: typical HUS (or D+ HUS) often seen in children as a result of a preceding Escherichia coli diarrheal illness, atypical HUS (or D− HUS) often seen in adults and caused by a variety of mutations of proteins in the complement cascade (factor H, factor I), and an autoimmune form of HUS characterized by antibodies to factor H or related proteins.
Factor H is a regulatory protein of the alternative complement pathway, and it functions to protect self-cells from attack by the complement system. Decreased levels of factor H, because of mutations or inhibitors, result in subsequent endothelial cell damage and thrombus formation at the site of exposed subendothelium. Similar to factor H is factor I, another regulator of the complement system. Factor I is a serine protease that cleaves C3b (an essential C3 cleavage product of the alternative complement pathway); its cofactors include factor H, C4-binding protein, and membrane cofactor protein (CD46). These abnormalities in the complement system have been theorized to be problematic for the renal vasculature, specifically because of the inherent presence of fenestrations within the capillary endothelium of the glomerulus, leaving the subendothelium more exposed and therefore vulnerable to complement attack.
The pathophysiologic mechanisms underlying D+ HUS are less well understood. A preceding diarrheal illness is often caused by E coli O157:H7, although other strains of E coli , Shigella , and Campylobacter have also been implicated because of the known production of the Shiga toxin. The Shiga toxin binds to Gb3, a glycosphingolipid receptor found on endothelial cells, podocytes, and tubular cells. There is evidence that the Shiga toxin induces the release of ultralarge vWF multimers from endothelial cells and inhibits their cleavage by ADAMTS13, resulting in enhanced platelet adhesion in in vitro experiments.
Diarrheal illnesses are not the only known infectious cause of HUS. Streptococcal pneumonia–related HUS is found in approximately 5% of children diagnosed with HUS and is presumed to be caused by the exposure of the Thomsen-Friedenreich (TF) antigen. This cryptic antigen is found on the surface of red cells, platelets, and glomerular endothelial cells and is normally hidden by neuraminic acid. Streptococcus sp produce the enzyme neuraminidase, thus allowing for the exposure of the TF antigen, which is then recognized by the immune system as foreign, triggering immune system response against those cells and leading to the development of HUS.
Histopathologic investigations of both TTP and HUS have been performed, and although these disorders often share a common clinical picture, the pathology of these 2 disorders are distinguishable. Autopsy specimens from patients with TTP contain platelet-rich thrombi within the microvasculature, whereas the tissues from patients with HUS have fibrin and red cell–rich thrombi. In addition, the frequency of which specific organs were found to have microthrombi was noted to be different in each of these diseases. The microthrombi of TTP were found in the heart, pancreas, kidney, adrenal gland, and brain. In HUS, these findings were primarily within the kidney and only infrequently found in the pancreas, adrenal gland, brain, and heart. This distribution of microthrombi speaks both to the clinical picture of these diseases and to the current understanding of the pathophysiology of each of these diseases, with TTP having a deficiency of circulating vWF cleaving protein leading to platelet microthrombi and multiorgan involvement and HUS resulting from a dysfunctional complement cascade, damage to renal vascular endothelium, and the triggering of thrombus formation through the coagulation cascade and subsequent acute renal failure.
Preeclampsia/HELLP
The pathophysiologic mechanisms behind the initial development of severe preeclampsia and HELLP are beginning to be better understood. Abnormal placental/maternal vascularization, inflammatory responses, and maternal hypercoagulability have each been theorized to play critical roles in the pathogenesis of these disorders.
Studies of the placentas from preeclamptic/HELLP pregnancies have shown abnormal vasculature development and areas of placental hypoperfusion and ischemia. Maternal-placental vascular endothelial dysfunction has also been demonstrated to play a central role. Both these events lead to platelet activation, which results in circulating microthrombi and organ damage/dysfunction.
Several placenta-derived factors have also been implicated in the development of preeclampsia, including the soluble Fms-like tyrosine kinase 1 (sFlt1) and soluble endoglin. Placenta-derived sFlt1 binds to circulating vascular endothelial growth factor (VEGF) and placental growth factor. These factors are essential for the normal development and vascularization of the placenta as well as normal glomerular endothelial function. In patients treated with VEGF antagonists, used in cancer therapy, hypertension and proteinuria have been reported. Soluble endoglin is also an antiangiogenic factor, inhibiting the normal binding of transforming growth factor βto its cellular receptors, leading to impaired capillary angiogenesis. In a study of pregnant rats, rats with elevation of either sFlt1 or endoglin levels had evidence of hypertension and proteinuria; however, in animals with increased levels of both factors there were significant nephritic levels of proteinuria, severe hypertension, elevated l -lactate dehydrogenase, abnormal liver functions, and thrombocytopenia, all clinical evidence of the development of HELLP syndrome. Increased levels of sFlt1 and soluble endoglin in pregnant women have also been shown to be predictive of the development of severe preeclampsia.
Beyond the antiangiogenic factors, numerous additional studies of gene expression profiling of placental tissue from preeclamptic and HELLP pregnancies have also implicated alterations in inflammatory responses. Increases in the levels of proinflammatory cytokines tumor necrosis factor α, interleukin (IL)-6, and IL-8 have been documented, as well as significant increases in leukocyte activation.
As a protective mechanism against delivery-associated hemorrhage, pregnancy is also a hypercoagulable state. Many alterations in factors involved in coagulation are observed in normal pregnancy, such as increased levels of fibrinogen; factor VII factor X, and FVIII; and vWF. Levels of fibrinogen, FVIII, and vWF can increase up to 3-fold by the third trimester. ADAMTS13 activity levels also decrease during pregnancy, averaging approximately 94% of the normal in the first trimester and 64% in the second and third trimesters.
The hypercoagulability of a normal pregnancy is enhanced in severe preeclampsia and HELLP not only due to endothelial damage and dysfunction leading to platelet activation but also due to elevated circulating levels of thrombomodulin, vWF, and plasminogen activator inhibitor-1. Additional studies comparing patients with severe preeclampsia and HELLP versus normal patients have shown that there is enhanced endothelial cell activation and decreased levels of ADAMTS13. ADAMTS13 activity is further reduced to a median of 31% in pregnant women with HELLP compared with 71% in normal pregnant women and 101% in nonpregnant women. While these changes result in an expected increased level of vWF in patients with both severe preeclampsia and HELLP as compared with normal healthy pregnant volunteers, ultralarge multimers are not found in these patients. The findings of decreased ADAMTS13 and increased vWF activity are supportive evidence that coagulopathic changes occur in these disease processes; however, these changes may not be causative and most likely represent consumption of the metalloprotease by ongoing thrombus formation and the vascular damage resulting in excessive release of vWF from endothelial stores.
Diagnostic evaluation
Outside the first trimester, in which preeclampsia and HELLP syndromes do not occur, the difficult task of distinguishing between preeclampsia, HELLP, TTP, and HUS can be both challenging and essential to obtaining a good maternal/fetal outcome. Obtaining rapid results of ADAMTS13 activity and von Willebrand multimeric analysis is unrealistic in most institutions, and the treating physician is left to make treatment decisions without these key pieces of information. Clinical features often widely overlap with each of these entities ( Box 1 ); however, treatment and subsequent outcomes are quite dependent on appropriate diagnosis. TTP is considered in patients presenting with severe thrombocytopenia, microangiopathic hemolytic anemia, neurologic abnormalities, and mild renal dysfunction (serum creatinine levels <3 mg/dL). HUS also presents with severe thrombocytopenia and microangiopathic hemolytic anemia; however, renal function is often more severely compromised with serum creatinine levels greater than 3 mg/dL, whereas neurologic abnormalities are less common. TTP-HUS can occur during any stage of pregnancy; however, data suggest that there is a higher incidence in the second and third trimesters, resulting in significant overlap with the preeclamptic disorders. Preeclampsia/HELLP may present with microangiopathic hemolytic anemia and severe thrombocytopenia is accompanied by hypertension and significant elevations in levels of liver enzymes. In preeclampsia/HELLP, these processes often resolve after delivery of the placenta. However, for patients in whom TTP/HUS is suspected, this prompt resolution will not occur.
TTP
- •
Thrombocytopenia (often severe <50,000)
- •
Hemolytic anemia
- •
Neurologic dysfunction
- •
Renal dysfunction (creatinine level <3mg/dL)
- •
Fever
- •
HUS
- •
Thrombocytopenia
- •
Hemolytic anemia
- •
Renal failure (creatinine level >3mg/dL)
- •
Hypertension
- •
Severe preeclampsia
- •
Hypertension
- •
Proteinuria
- •
Neurologic dysfunction (headache, blurred vision)
- •
Thrombocytopenia
- •
Hemolytic anemia
- •
HELLP
- •
Thrombocytopenia (often mild <100,000, but may be <50,000)
- •
Hemolytic anemia
- •
Liver dysfunction
- •
A better understanding of the pathophysiologic mechanisms underlying each entity has led to the study of ADAMTS13 and vWF multimers in patients with suspected severe preeclampsia and HELLP in an attempt to distinguish it from TTP. Whereas these patients do have mild reductions in ADAMTS13 and elevation in vWF, women with HELLP do not have ultralarge vWF multimers. Because none of the patients with HELLP had ultralarge vWF multimers, undetectable ADAMTS13, or presence of antibodies, TTP was ruled out in each of these cases; however, if a patient were to have an ADAMTS13 activity of less than 10%, TTP would have been the correct diagnosis.
Ultralarge multimers, however, may not always be found in patients with TTP. Initially, the vWF may be consumed in microthrombi, and once treatment starts and ADAMTS13 levels begin to increase, it may be cleaved as usual thus producing relatively normal-sized multimers.
Distinguishing TTP from HUS is often based on clinical presentation. However, patients with atypical HUS are more challenging. vWF multimeric patterns in patients with HUS often show decreases in large multimers, which is secondary to the enhanced sheer stress that the protein experiences in the microvasculature, whereas the ADAMTS13 levels and activity remain near normal. Adding to the complexity of this situation are studies documenting that the Shiga toxin can induce secretion of ultralarge vWF multimers from endothelial cells and simultaneously impair ADAMTS13 function.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree