Metastasis is defined as the spread of malignant cells from a primary tumor to a distant organ. It is estimated that 90% of all cancer deaths are a result of metastasis.1 Colorectal cancer (CRC) can metastasize to regional lymph nodes, liver, lung, or the peritoneal surface, and less frequently to other organs such as ovaries, brain, and bone. While CRC with locoregional lymphatic spread is categorized as stage III disease (5-year overall survival (OS) 70.4%), CRC with spread to distant organs is categorized as stage IV—or metastatic—CRC (CRCm) and carries a significantly worse prognosis (5-year OS 12.3%).2
Metastasis may be present at the time of diagnosis (synchronous) or may develop after treatment of the primary tumor (metachronous). Synchronous metastases are usually more extensive and portend a poorer prognosis. Metachronous metastases occur in as many as one-third of patients who were treated with apparent success for their primary tumor.
According to the Surveillance, Epidemiology, and End Results (SEER) database, 20% of CRC patients have metastatic disease at the time of diagnosis.2 Clinical presentation varies significantly, depending on the type and extent of disease spread. Patients with a single metastatic lesion are frequently asymptomatic. Others with more advanced disease involving several organs often present with pain, obstruction, or other debilitating symptoms.
Although the overall rate of cure is low, aggressive treatment is indicated for most patients with CRCm in order to extend survival and improve health-related quality of life (QOL). Treatment is complex and varies based on patient characteristics and the number, size, and location of metastatic lesions. Given the diversity of the metastatic load, and the multiple potential treatment alternatives (systemic or regional chemotherapy, radiation, surgery, endoscopy, ablation, and others), management of the patient with CRCm must be individualized on a case-by-case basis and developed within the context of a multidisciplinary setting. The goals, priorities, and anticipated course of treatment should be discussed not only with the disease management team but also with the patient and his or her family.
The development of metastasis is viewed as a continuous process that begins early in tumor formation and evolves as the tumor grows.3 It is thought that, after the initial malignant transformation, the tumor proliferates into a small mass of heterogenous cells with different metastatic potentials. These cells undergo a number of sequential genetic changes, characterized by the activation of oncogenes and inactivation in tumor suppressor genes. As the tumor grows beyond 1 mm in diameter and becomes relatively hypoxic, angiogenesis is initiated.4 Some tumors grow by utilizing other existing blood vessels in nearby tissues.
The detachment of tumor cells from the primary tumor mass requires the downregulation of cell adhesion molecules that would normally anchor them to other cells and the extracellular matrix. The best characterized alteration involves loss of E-cadherin, a key cell-to-cell adhesion molecule. This protein is essential in forming adherens junctions between adjacent epithelial cells, which facilitates the assemblage of epithelial cell sheets and maintains the quiescence of cells within these sheets. Conversely, adhesion molecules such as N-cadherin, normally associated with the cell migration that occurs during embryogenesis and inflammation, are often upregulated in many invasive carcinoma cells. A developmental regulatory program, referred to as the epithelial-mesenchymal transition (EMT), has been implicated as a means by which transformed epithelial cells acquire the ability to invade, resist apoptosis, and disseminate. A set of pleiotropically acting transcriptional factors, including Snail, Slug, Twist, and Zeb1/2, orchestrate the EMT and related migratory processes during embryogenesis. These factors are responsible for the loss of adherens junctions, and an associated conversion from a polygonal/epithelial to a spindly/fibroblastic morphology characterized by expression of matrix-degrading enzymes, increased motility, and heightened resistance to apoptosis—all traits that are implicated in the processes of invasion and metastasis.5
Circulating cancer cells invade target organs, where they form small micrometastases that, over time, may give rise to macroscopic tumors. These metastatic cells may become dormant or proliferate. What determines this is not fully understood. The ability of tumor cells to grow in the distant organ after deposition is a major limiting factor in the formation of metastasis, and some metastatic cells probably remain dormant for years. The majority of disseminated cancer cells are poorly adapted, at least initially, to the microenvironment of the tissue they invade. Accordingly, each type of disseminated cell may develop its own set of ad hoc solutions to the problem of thriving in the microenvironment of one foreign tissue or another.6 These adaptations may require hundreds of distinct genetic colonization programs, dictated by the cell type of the disseminating cancer, as well as the nature of the tissue microenvironment in which colonization occurs. Once deposited in a distant organ, the metastatic focus, if proliferative, must again go through tumorigenesis, angiogenesis, and evasion of the immune system.7
There appear to be genes specific to tumorigenesis, invasion, angiogenesis, and other processes. In fact, a number of genes have been identified that suppress metastatic potential through downregulation, affecting a cell’s ability to metastasize without affecting tumorigenicity.8 Identification and understanding of these mechanisms will have a profound impact on cancer treatment in the future.
As opposed to local or regional disease (stages I–III) in which surgery is the primary form of treatment, in the metastatic setting surgery is usually limited to patients with a small burden of disease (often localized to a single organ) or to patients with complications related to the primary tumor. With the development of new and more effective chemotherapeutic agents, many patients are now treated with a multimodality approach.
The initial evaluation of patients with metastatic disease is similar to that of any patient with colon or rectal cancer. Workup should include colonoscopy with biopsy, complete blood count (CBC), chemistry profile, and CT imaging of the chest, abdomen, and pelvis.9,10 The need for positron emission tomography (PET) scanning is debatable, but is useful in patients with doubtful lesions, elevated carcinoembryogenic antigen (CEA), and negative computed tomography (CT). It has been estimated that a PET-CT may affect patient management in up to 20% of patients with CRCm.11 In the absence of symptoms, there is no indication for brain imaging or bone scan. Patients with rectal cancer should also have locoregional staging with endorectal ultrasound or magnetic resonance imaging (MRI), as this information may be useful at the time of decision-making regarding treatment of the primary tumor. Ultimately, treatment will depend on the presentation of the disease. While a straightforward focus on the metastasis is the obvious approach in the setting of metachronous lesions, in synchronous cases the primary tumor status should also be taken into account.
Once the extent of disease workup is complete and distant metastases have been documented, the surgeon must make three important judgments. First: Is the patient fit for aggressive treatment? Patients with poor performance status or serious cardiovascular, pulmonary, renal, neurological, or gastrointestinal impairment may not tolerate chemotherapy or major surgery. Second: In the setting of synchronous tumors, does the primary tumor present a clinically significant risk of bowel obstruction, perforation, or bleeding? Symptoms, and radiographic and endoscopic findings, are important determinants of this eventuality. If the proximal colon is not dilated on radiographic studies, and a colonoscope can traverse the tumor, it is generally safe to begin chemotherapy. Third: Are the patient’s metastases amenable to resection and treatable with curative intent? If complete resection of all disease is anticipated, surgical intervention should assume a high priority.
A number of antineoplastic agents such as 5-fluorouracil/leucovorin (5-FU/LV) or capecitabine, oxaliplatin, and irinotecan, and new biologic agents such as bevacizumab, ziv-aflibercept, cetuximab, panitumumab, and regorafenib, are currently used in combination or as single agents in the treatment of stage IV CRC. The putative mechanism of action and the toxicity profile of each drug are presented in Table 115-1.
Chemotherapeutic Agents Commonly Used for Treatment of CRCm
| Agents | Mechanism | Common Side-Effects |
|---|---|---|
| 5-FU | Pyrimidine analog, thymidylate synthase (TS) inhibitor |
|
| Folinic acid (leucovorin) | Enhances 5-FU effects and contributes to TS inhibition |
|
| Irinotecan | Topoisomerase I inhibitor |
|
| Capecitabine | Pro-drug of 5-FU |
|
| Oxaliplatin | Platinum-based agent, DNA cross-linking |
|
| Cetuximab/panitumumab | Anti-EGFR monoclonal antibody (for use with KRAS wild-type tumors only) |
|
| Bevacizumab | Anti-VEGF monoclonal antibody |
|
| Regorafenib | Tyrosine kinase inhibitor | GI: diarrhea, weight loss, hepatotoxicity. Skin reactions. |
The choice of treatment regimen is based on the goals of therapy, the type and timing of previous therapy, and the toxicity profile of the constituent drugs.9,10 Chemotherapy regimens commonly used as first-line treatment in patients capable of undergoing intensive therapy include FOLFOX (5-FU/LV/oxaliplatin), FOLFIRI (5-FU/LV/irinotecan), CapeOx (capecitabine/oxaliplatin), infusional 5-FU/LV, capecitabine as a single agent, or FOLFIRINOX (5-FU/LV/oxaliplatin/ irinotecan). Cetuximab can be added to first-line therapy in patients with KRAS/NRAS wild-type CRC, and other combinations such as FOLFOXIRI with bevacizumab have shown promising results.12,13 Increasingly, the evidence suggests that selection of chemotherapeutic agents will be guided by molecular and genomic characterizations. Early reports indicate that combined therapy with BRAF and EGFR inhibitors may be effective in treating BRAF V600E metastatic CRCs specifically.14
The goals of chemotherapy depend on the clinical scenario, based on whether the disease is nonresectable or potentially resectable. Most patients fall into the first category, and chemotherapy in this context is palliative—the goal being to prolong survival while optimizing health-related quality of life (QOL). The model of distinct lines of chemotherapy (in which regimens containing non-cross-resistant drugs are each used in succession, until disease progression) is currently being abandoned in the setting of incurable stage IV CRC, and replaced with a continuum-of-care approach.15 This is an individualized strategy that includes phases of maintenance chemotherapy interspersed with more aggressive treatment protocols, as well as reutilization of previously administered chemotherapeutic agents in combination with other active drugs. The continuum-of-care approach is preferential because patients with nonresectable metastatic disease benefit more from access to all active agents. In these patients, inducing response is not as important as delaying tumor progression; achieving stability of disease may constitute successful treatment. The median survival of patients with unresectable stage IV CRC, which rarely exceeded 12 months when 5-FU/LV was the sole chemotherapeutic option, now exceeds 30 months when various agents are used in combination.
Selected patients with metastatic disease that is considered unresectable at the time of diagnosis may become surgical candidates if they achieve an adequate response to CT. This approach has been called conversion therapy to distinguish it from neoadjuvant treatment (in which metastases are already resectable upfront). Surgical outcomes and neoadjuvant/adjuvant chemotherapeutic strategies are discussed in the following sections.
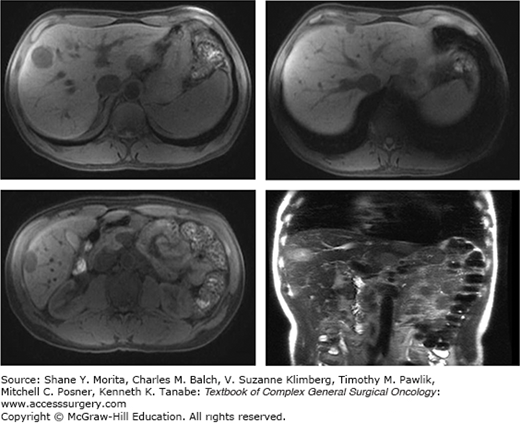
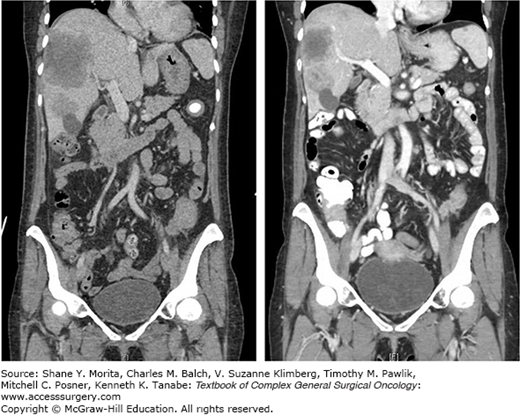
Approximately 60% of the 150,000 new cases of primary CRC diagnosed every year in the United States present with liver metastases, and one-third of these patients have liver-only disease16 (Fig. 115-1). The available regional treatments for CRC hepatic metastases include surgical resection, local tumor ablation (i.e., instillation of alcohol or acetic acid directly into the metastatic lesions, radiofrequency ablation (RFA), regional hepatic intra-arterial chemotherapy or chemoembolization, and radiation therapy (RT)). Of these, only surgery provides a survival benefit.
It is estimated that about 10% of all patients with colorectal liver metastases are candidates for potentially curative hepatic resection.17 Five-year survival in these patients is 30% to 45% (Table 115-2). The small percentage of patients with metastatic CRC who are cured by hepatic resection reinforces the importance of careful patient selection. These statistics also show that most patients with liver metastases are unresectable and require chemotherapy or supportive care. Nevertheless, with improvements in chemotherapy and in surgical and ablative techniques, the number of patients suitable for hepatic resection is growing.18,19
Morbidity and Survival after Hepatectomy for CRCm
| Authors | Year | Number of Patients | Morbidity (%) | Mortality (%) | Five-year OS (%) | Median Survival (Months) |
|---|---|---|---|---|---|---|
| Hughes et al135 | 1986 | 607 | – | – | 33 | NR |
| Scheele et al136 | 1995 | 434 | 16 (n = 67) | 4.4(n = 19) | 33 | 40 |
| Nordlinger et al137 | 1996 | 1568 | 23 (n = 345) | 2.3 (n = 36) | 28 | NR |
| Jamison et al138 | 1997 | 280 | – | – | 27 | 33 |
| Fong et al32 | 1999 | 1001 | 31 (NR) | 2.8 (n = 28) | 37 | 42 |
| Iwatsuki et al139 | 1999 | 305 | 8.2 (n = 25) | 0 | 32 | NR |
| Choti et al140 | 2002 | 133 | 23 (NR) | 0 | 58 | NR |
| Abdalla et al141 | 2004 | 190 | NR | NR | 58 | NR |
| Fernandez et al142 | 2004 | 100 | NR | 1 (n = 1) | 58 | NR |
| Wei et al143 | 2006 | 423 | 17 (n = 74) | 1.6 (n = 7) | 47 | NR |
| Rees et al144 | 2008 | 929 | 25.9 (n = 241) | 1.5 (n = 12) | 36 | 42.5 |
| de Jong et al145 | 2009 | 1669 | NR | NR | 47 | 36 |
| Morris et al146 | 2010 | 3116 | NR | NR | 44 | NR |
Information regarding the natural history of CRC liver metastases comes from reports published before the 1980s, when most hepatic metastases were left untreated. Several investigators have retrospectively studied untreated patients, reporting median survival of 5 to 10 months; long-term survival was rarely seen.20 A large proportion of these patients, however, had extensive disease, and most had their primary tumor in place, making any comparison to modern surgical series a difficult task. Some investigators retrospectively identified patients with isolated, potentially resectable hepatic metastases left untreated. In patients with metastases limited to the liver, who would otherwise be potential candidates for surgery, 3-year survival was 14% to 23% and 5-year survival was 2% to 8%.21,22 These studies demonstrated the relationship between bulk of disease and survival, and also clearly showed that—even in the best scenario—5-year survival of untreated patients is uncommon.
Although response rates to chemotherapy are improving, the only treatment ever shown to be potentially curative in CRC hepatic metastasis is complete resection. Modern multidisciplinary consensus defines resectable CRC liver metastases as tumors that can be completely resected, leaving sufficient liver remnant.23 In order to consider a patient for hepatic resection, there should be no radiographic evidence of hepatic artery, major bile duct or main portal vein involvement, or celiac/para-aortic lymph node enlargement.24 There should be no unresectable extrahepatic foci of disease, and the primary tumor must be resectable for cure.
Hepatic resection in the 1970s and 1980s was associated with high morbidity and mortality, making its role in the treatment of CRC liver metastasis at least questionable.25 Over the last 20 years, however, large series have demonstrated that liver resection can now be practiced with acceptable safety, and patients with isolated and resectable hepatic metastases have the potential for long-term survival (Table 115-2). In modern series, mortality is 5% or less; however, morbidity is still significant—reportedly between 20% and 50%. Nevertheless, this generally does not result in lengthy hospital stays, intensive care unit stays, long-term disability, or early mortality. The most serious complications, such as liver failure and hemorrhage, are now fairly uncommon, reflecting significant improvements in surgical technique and perioperative care. The survival results of surgery compare favorably to the results of no treatment (median survival 5 to 10 months) and chemotherapy (median survival 20 to 30 months). It is clear that complete resection still provides the best outcomes. While true long-term cure through chemotherapy is exceptionally rare, most long-term survivors following liver surgery are disease-free and presumably cured.26
The search for prognostic factors to facilitate selection of patients most likely to benefit from hepatectomy—and, more importantly, those who are unlikely to benefit—has been the subject of active research. The two most consistent contraindications to liver surgery are the presence of unresectable extrahepatic disease and the inability to achieve a complete resection. Other poor prognostic factors include involvement of lymph nodes, synchronous metastasis (or a short disease-free interval in patients with metachronous metastasis), a large number of lesions, bilobar hepatic metastases, and CEA elevation greater than 200 ng/mL.27–31
A multivariate analysis of 1001 patients at Memorial Sloan Kettering Cancer Center who underwent potentially curative hepatectomy identified five factors having the most influence on outcome.32 These included size greater than 5 cm, disease-free interval of less than 1 year, more than one tumor, a lymph node–positive primary, and CEA greater than 200 ng/mL. Using these variables, a risk score was developed that is predictive of recurrence following resection (Table 115-3).
Memorial Sloan Kettering Cancer Center Clinical Risk Scorea and Survival in 1001 Patients Undergoing Liver Resection for CRCm
| Score | 1-Year Survival (%) | 3-Year Survival (%) | 5-Year Survival (%) | Median Survival (months) |
|---|---|---|---|---|
| 0 | 93 | 72 | 60 | 74 |
| 1 | 91 | 66 | 44 | 51 |
| 2 | 89 | 60 | 40 | 47 |
| 3 | 86 | 42 | 20 | 33 |
| 4 | 70 | 38 | 25 | 20 |
| 5 | 71 | 27 | 14 | 22 |
The traditional approach to synchronous metastases has been resection of the primary tumor, followed by chemotherapy and metastasectomy. This approach has been criticized, however, because an initially resectable metastatic lesion might progress and become unresectable during convalescence of treatment of the primary tumor. To overcome this difficulty, the liver-first approach was proposed by Mentha et al.33 This consists of chemotherapy first, resection of liver metastases second, and, finally, removal of the primary tumor. In their original report, the authors described a median survival of 44 months, and OS rates at 1, 2, 3, and 4 years of 100%, 89%, 60%, and 44%, respectively.33 The current evidence is limited to small series of about 30 patients, with promising results; median survival of 27 to 69 months has been reported.34–36
The increased efficacy of systemic chemotherapy has prompted interest in treatment consisting of preoperative or neoadjuvant systemic chemotherapy prior to hepatic resection. This approach has a series of potential advantages, including earlier treatment of micrometastatic disease, the ability to evaluate tumor responsiveness, and the possibility of avoiding resection in patients demonstrating tumor progression during therapy (Fig. 115-2). The main disadvantages of preoperative chemotherapy are progression of nonresponsive tumors (which therefore become unresectable during systemic treatment) and an increase in perioperative morbidity due to chemotherapy-induced toxicity. A number of randomized controlled trials have found a modest improvement in relapse-free survival (RFS), but not OS, when chemotherapy is delivered before or after hepatic resection, compared with resection alone. The optimal timing of chemotherapy remains unknown.37
The EORTC Intergroup Trial is the only randomized trial to evaluate the role of perioperative FOLFOX (delivered 3 months before and 3 months after liver surgery) versus surgery alone in the management of resectable hepatic metastases. This study demonstrated an absolute increase of 7.3% in 3-year progression-free survival (PFS), favoring the combined treatment group.38 As expected, the trial also showed an increased rate of postoperative complications in the chemotherapy group (25%) compared with the surgery-alone group (16%), specifically a doubling in biliary fistula and hepatic failure rates; however, there was no significant difference in chemotherapy-related mortality (one vs. two patients). The study concluded that perioperative chemotherapy may improve cancer-specific outcomes, but at the cost of increased rates of postoperative complications, including liver dysfunction. Long-term follow-up revealed the same benefit in PFS, but no difference in OS.39
A limited number of patients present with liver-only metastasis not amenable to complete resection mainly because of proximity to essential anatomical structures. In this scenario, systemic chemotherapy aims to convert an unresectable patient to resectable status. Duration of treatment is critical. The minimum amount of time necessary to achieve a response should be weighed against the hepatotoxicity resulting from chemotherapy.
Both FOLFOX and FOLFIRI increase resectability in 12.5% to 40% of patients who are initially considered unresectable. One study demonstrated that adding irinotecan to FOLFOX (FOLFIRINOX) in patients with initially unresectable metastasis increased the resection rate, compared to FOLFOX alone.40 The addition of EGFR inhibitors to FOLFOX or FOLFIRI increased the resectability rate in patients with wild-type KRAS/NRAS tumors and initially unresectable liver metastasis.41,42 Angiogenesis inhibitors combined with FOLFIRI modestly increased the resectability rate of initially unresectable liver metastasis, compared to FOLFIRI alone, but had no effect when combined with oxaliplatin-containing regimens.43,44 To limit FOLFOX- and FOLFIRI-associated toxicity, patients should be evaluated for response every 2 months, and should undergo surgery as soon as their disease becomes resectable.
The role of chemotherapy following metastasectomy is not well-defined. A few trials have studied the benefit of systemic 5-FU-based chemotherapy following resection of hepatic metastases; however, clear evidence of a survival benefit, compared to observation alone, has not been shown.
In the early 1990s, two randomized controlled trials addressed the subject (the French Fédération Francophone de Cancérologie Digestive (FFCD) 9002 and European Organisation for Research and Treatment of Cancer/National Cancer Institute of Canada (EORTC/NCIC) trials), although both closed prematurely because of slow accrual.45,46 The FFCD-assigned 173 (of a planned 200) patients to 6 months of postoperative systemic 5-FU/LV versus observation alone, following hepatic metastasectomy. Patients receiving chemotherapy presented with a significantly better 5-year DFS as the primary endpoint (34% vs. 27%), but the trend was only toward better OS (51% vs. 41%). The EORTC trial used the same chemotherapy regimen, but its results have not been published separately. A pooled analysis of both trials, which included 278 patients, showed differences in median PFS (28 vs. 19 months, p = 0.058) and OS (62 vs. 47 months), although the evidence did not reach statistical significance.
Another trial from Japan randomly assigned 180 patients undergoing curative resection of CRC liver metastases to five 35-day cycles of adjuvant uracil-tegafur (UFT)/LV versus no adjuvant therapy.47 In a preliminary report, with a median follow-up of 4.76 years, adjuvant chemotherapy was associated with a significant improvement in 3-year relapse-free survival (RFS) (39% vs. 32%; HR for relapse 0.56, 95% CI 0.38 to 0.83), but no difference in OS (83% vs. 82%). These data constitute the basis for the recommendation of adjuvant chemotherapy after hepatic metastasectomy. However, an important consideration is that the chemotherapy used in these trials is inferior to current standards. Unfortunately, randomized trials show little evidence of benefits associated with the new schemes.
A randomized trial from the EORTC evaluated perioperative FOLFOX/chemotherapy (six cycles preoperatively, six postoperatively) versus observation in patients with initially resectable liver metastases, showing a trend toward improved 3-year PFS for chemotherapy versus surgical resection alone.38 However, in the most recent update of this trial, 5-year OS was not significantly better in the chemotherapy group (52% vs. 48%, HR for death 0.88, 95% CI 0.68 to 1.14), nor was there a significant difference when the analysis was restricted to CRC deaths.48 Unfortunately, as the trial was not powered for OS analysis, no conclusion can be drawn.
The addition of irinotecan to 5-FU and LV was studied in a multicenter trial of 321 patients undergoing complete surgical resection for liver-isolated metastatic disease, who were randomly assigned to short-term infusional 5-FU/LV with or without irinotecan.49 At a median follow-up of 42 months, the addition of irinotecan demonstrated no significant advantage in disease-free survival (DFS) (median 25 vs. 22 months).
The role of cetuximab was studied in a randomized trial from the EORTC evaluating perioperative oxaliplatin plus a fluoropyrimidine, with or without cetuximab, in patients with initially resectable liver metastases. In a preliminary report, the addition of cetuximab was associated with significantly worse PFS.50
Although the evidence is not strong, the National Comprehensive Cancer Network (NCCN) guidelines9,10 recommend a total of 6 months of perioperative treatment with an active systemic regimen for patients undergoing hepatic resection of stage IV CRC. In this setting there is no evidence to support the use of irinotecan or cetuximab; FOLFOX or XELOX is the preferred option.
Liver metastases measuring >0.5 cm receive more than 80% of their blood supply from the hepatic arterial circulation, whereas normal hepatocytes are primarily nurtured by the portal circulation.51 Administration of chemotherapy into the hepatic artery allows selective delivery of drug to tumor, sparing normal tissue. Depending upon a drug’s clearance and toxicity profile, a marked increase in its local concentration may be achieved by injecting it into the hepatic artery. Regional administration of drugs that are rapidly metabolized in the liver via a first-pass effect leads to higher levels of drug exposure, minimizing systemic side effects.
The goal of intrahepatic artery catheter placement is to enable bilobar hepatic perfusion of chemotherapy, preventing administration to the stomach or the duodenum (misperfusion). A preoperative angiogram is performed to define the arterial supply of the liver. If the vascular anatomy is suitable for placement of a hepatic artery catheter (hepatic arterial infusion pump (HAIP)), laparotomy is performed. If there is no contraindication to pump placement (such as extrahepatic disease) a cholecystectomy is performed. Total devascularization of the distal stomach and proximal duodenum is done in order to minimize the risk of misperfusion. In nearly all cases, the gastroduodenal artery is the vessel of choice for cannulation. Postoperatively, before initiating HIA chemotherapy, a Tc-99m macro-aggregated albumin (TcMAA) scan is necessary to ensure the absence of misperfusion and to assess the adequacy of whole liver perfusion. In order to achieve maximal clinical response, perfusion of the entire liver parenchyma is considered important. Early postoperative complications include arterial injury resulting in subsequent hepatic artery thrombosis, incomplete perfusion of the entire liver, misperfusion to the stomach or duodenum, and subcutaneus pocket hematoma. However, early complications are reported in fewer than 5% of patients. Late complications are more common, and include inflammation or ulceration of the stomach or duodenum, pump pocket infection, or catheter thrombosis. The development of antral or duodenal ulceration should prompt evaluation for misperfusion. There is also additional toxicity related to chemotherapy; in fact, early trials using high doses of floxuridine (FUDR) induced severe biliary toxicity. Biliary sclerosis is a result of direct toxic effect on the biliary tree, or ischemic changes, secondary to fibrosis, of the pericholangitic venous plexus, which ultimately leads to biliary stricture.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree