38 Metastatic Brain Tumors
Brain metastases, aside from being the most common brain tumors, are one of the most feared consequences of systemic cancer because of their poor prognosis if left untreated. Although 10 to 30% of cancer patients ultimately develop brain metastases, the incidence is increasing due to increased cancer survival.1 Treatment of brain metastasis consists of surgical resection, radiation therapy, or a combination of the two. With advances in surgery and stereotactic radiosurgery (SRS), therapeutic options have increased, and long-term survival has become a reasonable goal. When to use the available treatments has been the source of considerable debate. This chapter reviews the therapeutic options and provides a rational basis for their appropriate application.
 Epidemiology
Epidemiology
The incidence of brain metastasis is estimated to range from 21,000 to over 100,000 new cases per year2 in the United States and is thought to be increasing with improved cancer survival, an aging population, increased awareness of the disease, and better diagnostic tests.1
Overall, lung cancer is the most common source of brain metastasis, and is responsible for over half of all cases, followed by breast cancer, melanoma, renal cell cancer, and colorectal cancer.1 Whereas the lung is the most common primary cancer source in men, breast cancer predominates in women. However, the percentage of patients with brain metastases is highest in patients having melanoma, with 40 to 60% developing brain metastases,3 and 60% percent of brain metastases occur in patients who are 50 to 70 years old.4
Based on magnetic resonance imaging (MRI) studies, 50 to 80% of brain metastases are multiple, whereas autopsy studies report that 60 to 85% of brain metastases are multiple. The relative frequency of multiple metastases varies with the type of primary tumor. Melanoma has the highest tendency to produce multiple lesions,5 whereas renal cancer metastases are more often single lesions.1
Pearl
• Any primary cancer can metastasize to the brain, and there are multiple reports in the literature of brain metastases from rare or unusual systemic tumors. Consequently, metastasis should always be considered in the differential diagnosis of a brain lesion in a cancer patient.
 Pathology
Pathology
Although on gross examination most metastases are spheroid and well demarcated from surrounding brain tissue, on microscopic examination these tumors may have an infiltrative appearance.1 Histologically, they appear similar to those of the primary lesion or to the systemic metastases that arise from the primary. Metastases are most often located at the junction of the gray and white matter of the brain, where tumor emboli are trapped in the cerebral vasculature. The cerebrum is the site of localization of 80 to 85% of brain metastases, the cerebellum 10 to 15%, and the brainstem, 3 to 5%.4
Special Consideration
• Although metastases are infiltrative much less often than gliomas, this quality may account for tumor recurrences and may justify the use of postoperative irradiation.
 Clinical and Imaging Features
Clinical and Imaging Features
Up to two thirds of all brain metastases are symptomatic at some time during a cancer patient’s life. The signs and symptoms of metastatic tumors are the same as the signs and symptoms of other expanding intracranial mass lesions.6 In patients with known systemic cancer, the appearance of neurologic symptoms and a lesion evident on imaging consistent with brain metastasis are virtually diagnostic. Of patients with a history of cancer, 89 to 93% who present with a single supratentorial lesion have a brain metastasis.7 In patients without a diagnosis of systemic cancer who have a single brain lesion with the imaging features of a metastasis, the probability is less than 15% that the mass is a metastasis.
Contrast-enhanced MRI is the single best tool for imaging evaluation of patients with suspected brain metastasis. MRI is more sensitive and specific than computed tomography (CT) in determining presence or absence, location, and number of metastases. On T1 MRI, metastases appear as loci of increased signal intensity. Larger tumors often appear to have peripheral enhancement with a nonenhancing core, representing central necrosis. Peritumoral edema appears on a T1 image as a region of decreased signal intensity. In T2 images, tumors often have decreased intensity, whereas edema appears as increased intensity. The presence and extent of edema are far better appreciated on T2 than on T1 imaging.
• Most patients with a single brain lesion and no history of cancer present with neurologic symptoms. Surgical intervention is usually required to relieve these symptoms. Thus, for these patients, whether a lesion should or should not be biopsied first is often not an issue.
Pitfall
• Unlike MRI, the CT scan cannot be relied on to determine the number of brain metastases present in a patient. Also, as MRI slice number is increased, by decreasing slice thickness and the space between slices, the chance of finding small metastases is increased.
 Treatment
Treatment
Patients with symptomatic brain metastases receive corticosteroid therapy initially, which decreases edema and often alleviates neurologic symptoms. Treatment of brain metastasis consists of surgical resection, radiation therapy, or a combination of the two. Determining which of these modalities is best for a particular patient depends on the number, size, and location of the lesion(s), the status of the systemic disease, the general health and neurologic condition of the patient, and the radiosensitivity or chemosensitivity of the lesion. The status of the systemic disease, both the primary tumor and non-cerebral metastases, is a particularly important determinant of outcome in patients with cerebral metastases. In general, patients with controlled primary tumors who have few or no noncerebral metastases are expected to have the best outcome and should be treated with the goal of local cure. More palliative approaches with limited risk for immediate morbidity should be considered for patients who have advanced primary tumors and multiple systemic metastases.
Whole-Brain Radiation Therapy
The use of whole-brain radiation therapy (WBRT) for the treatment of brain metastasis was first reported in 1954.8 Since then, numerous studies have analyzed the role of WBRT in the treatment of brain metastases.1 The advantage of WBRT is that it is a simple, noninvasive method of treating the entire brain. Unlike local treatments such as surgery or SRS, WBRT can control metastatic deposits throughout the brain, particularly small or microscopic ones. Consequently, WBRT is best suited for patients with multiple brain metastases. The effectiveness of WBRT depends at least in part on the histology of the lesion. Although breast and lung cancers may respond favorably, tumors such as melanoma and renal cancer are more radioresistant.
The major disadvantage of WBRT is that the normal brain is exposed to the effects of ionizing radiation, which may result in untoward side effects depending on the total dose, fraction size, and dosing interval. Acute side effects include dry desquamation, hair loss, headaches, nausea, lethargy, otitis media, and brain edema. A “somnolence syndrome” of increased fatigue can appear 1 to 4 months after treatment. Late effects can be more serious and include radiation necrosis, atrophy, leukoencephalopathy, and dementia.1 Large daily radiotherapy fraction sizes have been shown to increase the risk of neurocognitive deficits.9 In patients with the potential to survive for longer than 1 year, radiation injury to the central nervous system can be a significant problem, and more attention to the potential cognitive effects of WBRT is warranted.10
Many series have shown that a median survival time of 3 to 6 months can be expected after WBRT depending on the number of lesions, their radiosensitivity, and the status of any extracranial lesions present. The Radiation Therapy Oncology Group (RTOG) has analyzed the effectiveness of various treatment schedules.1 These studies indicated that 30 Gy delivered in 10 fractions over 2 weeks results in a rate and length of palliation equivalent to more protracted and higher dose schedules. Yet the risk of radiation-induced leukoencephalopathy, as a consequence of damage to microvessels, increases with radiotherapy fraction sizes > 2 Gy.11 Consequently, at The University of Texas M.D. Anderson Cancer Center, patients with favorable prognostic factors are frequently treated with 15 fractions of 2 Gy to a total dose of 30 Gy instead of the more customary 10 fractions of 3 Gy.
Whole-brain radiation therapy may be used as primary therapy or as adjuvant treatment after surgical resection or SRS. As primary therapy, WBRT should be considered for all patients with multiple brain metastases and for all patients whose tumors are highly radiosensitive. For patients with single brain metastases, surgery or SRS are probably better options, except in patients with highly radiosensitive tumors such as small-cell lung cancer or germ-cell tumor metastases, in whom WBRT is the best option. In fact, in a randomized trial, prophylactic cranial irradiation has been shown to reduce the incidence of symptomatic brain metastasis and to improve disease-free and overall survival for patients with small-cell lung cancer.12
Whole-brain radiation therapy may be a better option than surgery or SRS in patients whose condition makes them a high risk for surgery or SRS. As an adjunctive treatment, patients undergoing surgery or SRS are often given WBRT afterward in an attempt to reduce the possibility of recurrence.
• Whole-brain radiation therapy is generally recommended for patients with multiple brain metastases. For patients with single brain metastases, WBRT may be used as the primary therapy when the tumor is radiosensitive, when the patient has advanced uncontrolled systemic disease, or for patients who cannot tolerate other treatments.
Surgical Resection
Although surgical resection was initially viewed with much nihilism, in the modern era, surgery has become an important treatment option. Surgical resection has several advantages over WBRT or SRS: (1) Surgery is the only modality that establishes a histological diagnosis. This is important because 5 to 11% of patients with single brain lesions and known systemic cancer have lesions that are not metastases.7 (2) Surgery rapidly relieves symptoms by reducing intracranial pressure, relieving local compression, and eliminating the source of edema. This reduces the need for prolonged steroid administration and lessens the incidence of associated complications that may occur with WBRT or SRS. (3) Unlike SRS, conventional surgery is effective against large tumors (> 3 cm in maximal diameter). The disadvantages of surgery are that it is invasive and has inherent morbidity.
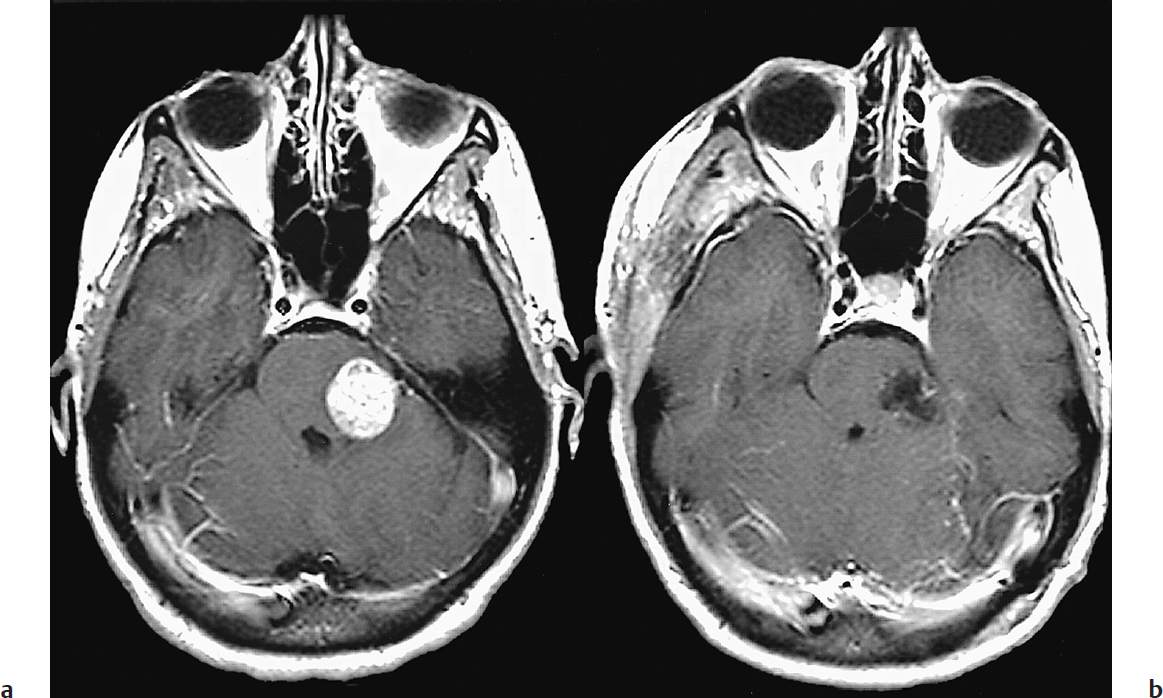
Surgical resection of single brain metastases is associated with a median survival time of 8 to 16 months and local recurrence rates of 7 to 15%.13,14 Modern techniques of microsurgery, computer-assisted stereotaxy, intraoperative ultrasonography, cortical mapping, and a better understanding of surgical approaches have made most lesions amenable to surgery, including those in deep locations (Fig. 38.1).13 These techniques have reduced the surgical morbidity to about 10% and mortality to less than 5%.
Nevertheless, the limited survival time of patients with systemic cancer demands that patients have short postoperative recovery times and incur no neurologic deficits that necessitate extensive rehabilitation. Therefore, metastases in the basal ganglia, thalamus, and brainstem are not usually resected. When reviewing our experience in patients who underwent surgery alone without WBRT, for a single, previously untreated brain metastasis, we identified two factors that independently affected local recurrence: tumor volume and method of resection.14 En bloc resection decreases the risk of leptomeningeal dissemination and local recurrence relative to piecemeal resection, particularly in tumors with a volume < 9.71 cm3,14–16 without incurring increased risk of neurologic deficit, even in eloquent areas.
Controversy
• Until the 1990s, the role of surgical resection versus WBRT for treatment of single brain metastases was an area of considerable debate, but now surgical resection is often the preferred initial option.
Fig. 38.1a,b (a) Preoperative and (b) postoperative contrast-enhanced, T1-weighted magnetic resonance imaging (MRI) scans of a 35-year-old woman with breast cancer metastatic to the pons. The patient was discharged on the fourth postoperative day, fully capable of independent living. (From Sawaya R. Surgical treatment of brain metastases. Clin Neurosurg 1999;45:41–47. Reproduced with permission.)
• Based on separate prospective randomized trials, surgical resection is the preferred approach for treating patients with single brain metastases.
In general, surgery is most appropriate for patients with a single brain metastasis, limited systemic disease, and a favorable Karnofsky Performance Scale (KPS) score (typically ≥ 70). Unless they are highly radiosensitive, most single lesions that are ≥ 3 cm in maximal diameter should be resected, as both SRS and WBRT have limited effectiveness against large tumors.
Although many retrospective studies suggest that surgery offers longer survival than WBRT, proponents of WBRT argue that surgical candidates are a select group whose long-term outcome depends on their good functional status rather than treatment with surgery. In 1996, a prospective randomized trial reported that surgery followed by WBRT did not show a survival advantage over WBRT alone in patients with single brain metastases17 (Table 38.1), but 73% of their patients had advanced extracranial systemic cancer. In contrast, two other prospective randomized trials7,18 (Table 38.1) in the 1990s demonstrated that surgery plus WBRT was superior to WBRT alone. In both studies, patients with single brain metastases, KPS scores ≥ 70, and limited systemic disease, who were treated with surgery, lived significantly longer, had fewer recurrences, and had a better quality of life than the patients treated with WBRT alone.
Surgery has traditionally been contraindicated once multiple lesions are identified.19 However, a retrospective review of patients harboring multiple brain metastases, comparing patients who had three lesions or less and underwent resection of either all lesions or just some of the lesions showed that patients with multiple metastases that were all resected survived significantly longer (median 14 months) than patients in whom at least one lesion was left unresected (median 6 months).20
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree