Menopause and Midlife Health Changes: Introduction
The profile of female geriatric patients will be changing considerably over the next decade. A substantial number of women born during the baby boom following World War II are at or beyond midlife, resulting in an increasing number of women who will be seeking treatment for symptoms associated with menopause and for chronic conditions that have their origin in midlife (see Chapter 5 for details on demographics). Further, the cohort of U.S. women who are now at midlife is unique. More than three-quarters are in the workforce; more than one-third have college degrees; and family composition has changed remarkably as evidenced by the number of live births that have declined by almost half.
For many of these women, the midlife, which encompasses the menopausal transition, will be a significant milestone and a harbinger of their health status and their interaction with health care systems for the ensuing decades. Thus, it is important to understand the events of the menopause transition and that these events are likely to affect health, the perception of health, and the perception of the contribution of the health care provider to health maintenance.
The initiation of the menopause transition (that time from active reproduction to the cessation of significant estrogen secretion because of the depletion of functional ovarian follicles) is an ill-defined period that commences with the onset of menstrual irregularity or skipped menses and ends 12 months following the final menstrual period (FMP). The median age at FMP is currently estimated to be 51.4 years. The menopause itself is the permanent cessation of menses and is clinically diagnosed following 12 continuous months of amenorrhea.
This geriatrics textbook includes a chapter on menopause because exposure to declining levels of ovarian hormones appears to modify risk factors associated with the development of debilitating diseases and health concerns of the geriatric patient. Furthermore, the menopause transition may represent an optimal time for clinical intervention. These clinical interventions can address two potential patient groups: (1) the 12% to 25% of women who already have some evidence of disability (see the physical limitations section) and for whom active interventions are critical and (2) the majority of women who are not disabled and are open to information about preventive care, risk factor screening, and maybe seeking care for menopausal symptoms.
Physiological Basis of Reproductive Aging
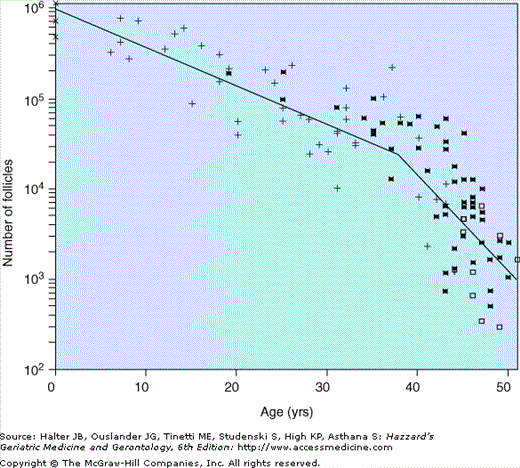
Aging of the female reproductive system is unique because the timeframe for the permanent cessation of menses has changed little over time, while the average life expectancy of women now extends to more than 30 years after the FMP. Further, women have a finite period of reproductive capacity. Unlike men, who usually retain reproductive capacity throughout their lifetime, women are born with a set number of primordial follicles, whose depletion begins prior to birth (Figure 46-1). At menopause, when a woman’s ovarian reserve is depleted, the number of available follicles recruited to form preovulatory follicles has dwindled to a point where ovarian-based hormones, including estrogens and progesterone, are no longer predictably produced. A progressively greater rise in gonadotropin, such as follicle stimulating hormone (FSH), is observed with the diminution of the follicular pool and the increasing nonresponsiveness in regulation of hormone secretion.
Figure 46-1.
Biexponential model of declining follicle numbers in pairs of human ovaries from neonatal age to 51 yrs old. Data were obtained from the studies of Block (1952, 1953) (X, n = 6; +, n = 43), Richardson et al. (1987) (□, n = 9) and Gougeon (1987) (*, n = 52). (Faddy MJ, Gosden RG, Gougeon A, et al. Accelerated disappearance of ovarian follicles in mid-life: implications for forecasting menopause. Human Reprod. 7:1342–1346, 1992. With permission from Oxford University Press.)
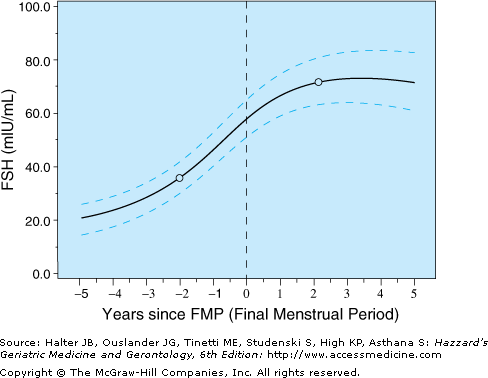
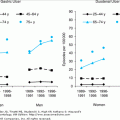
It is likely that changes in lipid, bone, or immunological functioning, among others, are related to those marked changes in estradiol and FSH concentrations during the menopause transition and occur when the hormone changes around the FMP are most pronounced. The Michigan Study of Women Across the Nation (SWAN) evaluated FSH values in relation to time and identified that there was an accelerated rise in FSH levels between 5 and 2 years prior to the FMP. FSH levels further accelerated from 2 years prior to 1 year after the FMP; thereafter, the FSH levels tended to plateau (Figure 46-2).
Figure 46-2.
Average FSH values (with 95% confidence intervals from bootstrapping) in relation to final menstrual period (FMP). (Sowers M, Zheng H, Tomey K et al. Changes in body composition in women over six yrs at mid-life: ovarian and chronological aging. J Clin Endocrin Metab. 2007;92:895–901. Copyright 2007, The Endocrine Society.)
Because of the complexity and the cost of measuring these hormone-driven menopausal transition events in the clinical setting, progress through the transition is frequently estimated with menstrual cycle characteristics. In 1996, the World Health Organization suggested definitions of the stages describing the transition to natural menopause. A broader and simpler staging system, including earlier stages of reproductive aging and later stages of postmenopause, was proposed at the International Stages of Reproductive Aging Workshop (STRAW). The STRAW staging definitions are based primarily on bleeding changes with the acknowledgment that discriminating hormone levels, specifically FSH, would add precision when sufficiently described (Table 46-1).
World Health Organization (WHO) |
Natural Menopause |
The permanent cessation of menstruation resulting from the loss of ovarian follicular activity. It is recognized after 12 consecutive months of amenorrhea, for which there is no other pathological or physiological cause. An adequate independent biological marker for the event does not exist. |
Perimenopause |
The period when the endocrinological, biological, and clinical features of menopause begin through the first year after the menopause. |
Menopausal Transition |
The period of time before the final menstrual period (FMP) when variability in the menstrual cycle is usually increased. |
Premenopause |
The period ambiguously referred to as either the 1 to 2 yrs immediately before the menopause or the whole of the reproductive period prior to the menopause. It is recommended that this term be used consistently in the latter sense to encompass the entire reproductive period up to the FMP. |
Induced Menopause |
The cessation of menstruation following either surgical removal of both ovaries or iatrogenic ablation of ovarian function. |
Simple Hysterectomy |
At least one ovary is conserved. It is used to define a distinct group of women in whom ovarian function may persist for a variable period after surgery. |
Postmenopause |
The period dating from the FMP, regardless of whether the menopause was induced or spontaneous. |
Premature Menopause |
Menopause that occurs at an age less than two standard deviations below the mean estimated for the reference population. In the absence of reliable population estimates, 40 yrs is frequently used as an arbitrary cut-off point. |
Stages of Reproductive Aging Workshop (STRAW) |
Reproductive Stage |
The period from menarche to the beginning of the perimenopause. |
Menopausal Transition |
The time of an increase in follicle-stimulating hormone and increased variability in cycle length, two skipped menstrual cycles with 60 or more days of amenorrhea or both. The menopausal transition concludes with the final menstrual period (FMP) and the beginning of postmenopause. |
Postmenopause |
Begins at the time of the FMP, although it is not recognized until after 12 months of amenorrhea. |
Based on menstrual calendar diary data of women undergoing the menopause transition, cycle lengths in excess of 60 days are a sensitive indicator of an impending FMP. This represents a pragmatic marker for use by patient and clinician alike in characterizing the menopause status of the woman. Further, these timeframes are consistent with our understanding that there are two follicle pools, one recruitable pool in which follicles, when stimulated, are immediately available for growth and a second pool of follicles insensitive to gonadotropin signals. The growable pool is refreshed approximately every 60 to 90 days. As the follicle supply decreases, the growable pool of follicles can no longer replenish itself to those numbers seen in mid-reproductive life. As the follicle reserve becomes critically diminished and few remaining responsive follicles are present, menstrual cycles become more irregular and are more likely to be anovulatory.
Aging of the reproductive system is frequently only considered in the context of the ovary; however, it actually involves all three levels of the reproductive axis—the brain, pituitary, and the ovary. While the hypothalamus and pituitary play extensive roles in all aging processes, the exact mechanisms by which gonadotropin releasing hormones (GnRH) change with age with respect to biosynthesis, transport, release, and receipt of feedback from neurotransmitters or steroids is incompletely understood. The total numbers of GnRH neurons do not appear to be the critical factor in determining reproductive senescence. Rather, changes in intermittent hormone secretion (i.e., luteinizing hormone (LH) or FSH) or glycoprotein free α-subunit (FAS) in pre- versus postmenopausal women result in a change in gonadotropin levels, characterized as a change in their pulse amplitude and, possibly, their pulse frequency. These changes in pulsatile release may reflect an unappreciated aging process or a greater insensitivity to feedback during the menopause transition.
In the aging hypothalamus, dopamine secretion into the portal vasculature declines and, with aging, the stimulatory action of estrogen is absent. In addition to dopamine, it is thought that norepinephrine, acting primarily through the α-adrenergic receptor, stimulates GnRH release, a process that declines with aging. This aging phenomena may explain why some of the symptoms commonly associated with the menopause transition (including potential cognitive changes) are more likely to reflect chronological change while other symptoms, such as vasomotor symptoms, appear to be more reflective of the events of change in ovarian hormone concentrations.
The onset or exacerbation of a constellation of symptoms, including vasomotor problems, vaginal dryness and other sexual symptoms, urine leakage, changes in skin, fatigue, and problems with sleeping, negative mood, and decline in cognitive acuity, is frequently attributed to the menopause. Further, many women choose to begin hormone replacement therapy in an effort to combat these menopausal symptoms and to replace the endogenous hormones that have been declining. These symptoms are described below in relation to their development, frequency, and degree of bother during the menopause transition and, when relevant, the effect of hormone therapy (HT).
Menopause Symptoms and Clinical Features
Vasomotor symptoms, including hot flashes, cold sweats, or night sweats, have been consistently associated with the menopause transition, occurring to some degree in more than 70% of women during midlife, with more than 50% of women reporting their presence for more than five years. Vasomotor symptoms are defined physiologically as a disruption in the hypothalamic central thermoregulation, causing the core body temperature to change by at least 0.2 degrees Celsius. In SWAN, the pattern of increasing perimenopausal hot flashes compared to the premenopause was consistent among African-American, Japanese, and Hispanic women. More vasomotor symptoms are found in those with increasing body mass index (BMI), current smoking status, baseline depression, and premenstrual symptoms. In a study evaluating symptom constructs in more than 500 Caucasian women, increasing frequency and amount of bother from vasomotor symptoms and sexuality were predicted by the transition from a premenopause to a postmenopause state over time. Low estradiol concentrations and higher FSH concentrations were significantly predictive of having vasomotor and sexual symptoms, but not other symptoms commonly identified with the menopause transition including urinary leakage and negative moods. Consistent with the findings from SWAN, this study found that higher BMI and current smoking behavior were highly related to increased bother with many symptom constructs, but especially vasomotor symptoms.
Investigators at the 2005 National Institute of Health (NIH) State-of-the-Science Conference, Management of Menopause-Related Symptoms, reported that sleep problems are identified by 16% to 42% of premenopausal women, 39% to 47% of perimenopausal women, and 35% to 60% of postmenopausal women. Although aging, in general, is associated with decrements in sleep quality, gender differences in sleep disturbances that emerge at midlife suggest that the increase in sleep difficulties may not be simply an aging effect. However, the majority of published studies are limited by small sample sizes, cross-sectional data, and limited assessments of sleep and menopausal status. In the SWAN multiethnic sample of 12 603 Caucasian, African-American, Chinese, Japanese, and Hispanic women, aged 40 to 55 years, almost 40% reported difficulty in sleeping. Age-adjusted rates for difficulty in sleeping were highest in the late perimenopause (45%) and in women with surgical menopause (48%). Menopausal status was associated with greater difficulty in sleeping, even following adjustment for age, race/ethnicity, vasomotor and psychological symptomatology, self-perceived health status and health behaviors, arthritis, and education, all factors that were also associated with sleeping difficulty (Table 46-2).
WOMEN WITHOUT VASOMOTOR SYMPTOMS (n = 7338) | ||||
|---|---|---|---|---|
ALL WOMEN (n = 11 222) | ||||
MENOPAUSAL STATUS | Odds Ratio | 95% CI | Odds Ratio | 95% CI |
Premenopausal | 1.00 | Reference | 1.00 | Reference |
Early perimenopause | 1.11 | 0.99–1.24 | 1.10 | 0.96–1.26 |
Late perimenopause | 1.33 | 1.07–1.65 | 1.40 | 1.01–1.92 |
Natural postmenopause | 1.21 | 1.03–1.43 | 1.26 | 1.01–1.58 |
Surgical postmenopause | 1.55 | 1.25–1.92 | 1.19 | 0.87–1.62 |
Postmenopause with hormones | 1.12 | 0.95–1.31 | 1.03 | 0.83–1.27 |
Factors that might trigger acute sleep disturbances during the menopause transition may include the onset and exacerbation of vasomotor symptoms, rate of changing hormone levels (especially FSH), and increases in symptoms of stress associated with acute and chronic ongoing life events. There is considerable overlap between sleep disturbances and vasomotor symptoms. A study by Dzaja et al. found that 68% to 85% of symptomatic menopausal women have vasomotor symptoms and 51% to 77% report insomnia.
In some studies comparing HT users and nonusers, HT users report more sleep disturbances suggesting that preexisting sleep disturbances influence the association between HT and the exacerbation of sleep disturbances. In a longitudinal study, Matthews et al. found that twice as many incident sleep complaints (28%) occurred among HT users compared to nonusers (14%). The greater increase in sleep problems among women who used HT may have been because of the preexisting characteristics of the women or to particular HT formulations or doses.
Worsening sleep during the menopausal transition is important because this sleep is associated with decreases in quality of life and poorer work performance. Persistent insomnia has been associated with an increased incidence of mood and anxiety disorders. Further, subjective sleep complaints and polysomnography-described sleep disruptions are associated with many negative health outcomes, including susceptibility to upper respiratory tract infections, immunosuppression, cardiovascular disease (CVD), and stroke and their attendant health care costs.
Sex steroids are postulated to affect sexual function (desire, arousal, orgasm, and pain) either directly through their neurohormonal effects on pain or desire or indirectly by maintaining the anatomic competence that allows arousal to occur. Atrophic changes in the vagina, including a decrease in lubrication accompanying sexual arousal, thinning of the endothelium, and loss of rugation, which are associated with dyspareunia have been well described. Because the menopausal transition is a time of significant change in sex steroid hormones and in sexual function, it has been speculated that the menopause leads to a diminution in sexual functioning, apart from the simultaneous potential effects of normal aging. However, the evidence linking sexual function to individual endogenous hormones or changes in hormones over time remains largely circumstantial.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree