Figure 143.1 Rates of meningococcal disease by age group.
However, there are four known situations where antibodies may not be protective: (1) individuals with complement component or properdin deficiencies are at an increased risk for developing invasive meningococcal infections because their serum loses the ability of complement-antibody-mediated lysis (bactericidal activity) of the meningococcal organism; hence, complement deficiency, most often of the terminal components, or properdin deficiency should be considered in persons with recurrent episodes of invasive meningococcal infection; (2) asplenic individuals are also at increased risk for suffering invasive meningococcal disease because of decreased efficiency of clearing of the encapsulated invading microorganisms from the blood; on rare occasions, persons may (3) develop serum immunoglobulin A (IgA) antibodies that block the bactericidal action of immunoglobulin G (IgG) and immunoglobulin M (IgM) antibodies or (4) possess genetic upper airway surfactant protein mutations that result in an impaired local first-line innate immune defense. All of these individuals should be periodically vaccinated (see below) to maintain as high a titer of anti-meningococcal antibodies as possible.
Clinical features
As with most other invasive gram-negative bacteria, the clinical consequences of meningococcal infection are primarily the result of meningococcal endotoxin (lipopolysaccharide) release and subsequent activation of the procoagulation, anticoagulation, fibrinolysis, complement, and kallikrein–kinin cascades resulting in the excessive release of various inflammatory mediators (“cytokine storm”). The clinical manifestations of meningococcal infection range from a mild transient bacteremia to fulminant meningococcemia, also referred to as Waterhouse–Friderichsen syndrome or purpura fulminans (Figure 143.2), with or without concurrent bacterial meningitis. Unless treated early, the mortality rate of the latter is high.
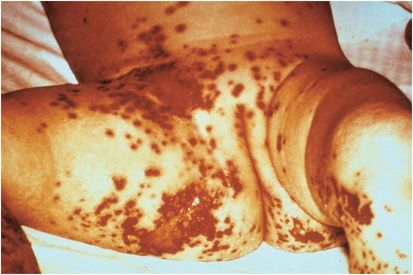
Figure 143.2 Purpura fulminans (Waterhouse–Friderichsen syndrome or severe ecchymotic rash).
Most often, Neisseria meningitidis acquisition results in asymptomatic colonization of the nasopharynx and oropharynx. The mildest form of invasive disease, a transient bacteremia, begins insidiously with fever, malaise, and symptoms of an upper respiratory tract infection. A few petechial skin lesions may appear, but neither signs nor symptoms of sepsis or meningitis develop. Signs and symptoms usually resolve spontaneously within 24 to 48 hours. However, the transient bacteremia may progress to acute meningococcemia heralded by fever, chills, malaise, weakness, headache, myalgias, and nausea and/or vomiting.
Skin manifestations, especially a petechial rash (Figure 143.3), should raise the index of suspicion for invasive meningococcal infection. They commonly appear in crops on the ankles, wrists, axilla, arms, legs, trunk, and mucous membranes, whereas the palms, soles, neck, and face are usually spared. The rash may also be urticarial, maculopapular, ecchymotic, or gangrenous depending on the degree of vascular pathology. In severe invasive meningococcal disease, the rash rarely fails to develop. Fulminant meningococcemia complicates acute meningococcemia in 5% to 15% of cases and is associated with the rash progressing into massive skin and mucosal hemorrhage, disseminated intravascular coagulopathy (DIC), and vascular collapse (Waterhouse–Friderichsen syndrome). Adrenal hemorrhage may occur despite appropriate antibiotic therapy.
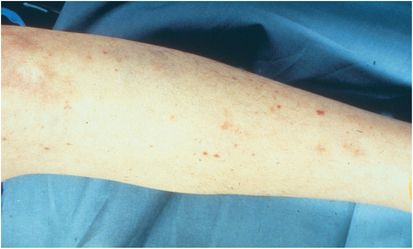
Figure 143.3 Petechial rash lower extremity.
Meningitis usually occurs along with the manifestations of meningococcemia but not always. Clinically, meningococcal meningitis resembles acute meningitis of any cause, presenting with fever, headache, altered sensorium/cognition, and nuchal rigidity.
On rare occasions, a chronic meningococcemia develops. This is characterized by intermittent febrile episodes, lasting 2 to 10 or more days, accompanied by a variety of skin lesions (macular, maculopapular, petechial, ecchymotic, or pustular), arthralgias or arthritis, myalgias, and splenomegaly. This manifestation may last for months and is sometimes fatal, but it usually resolves spontaneously. Occasionally, N. meningitidis may cause oropharyngitis, sinusitis, pneumonia, conjunctivitis, endophthalmitis, proctitis, urethritis, cervicitis, immune-mediated arthritis, endocarditis, myocarditis, pericarditis, or pelvic inflammatory disease (PID). Nonspecific helpful laboratory abnormalities include an elevated white blood cell count, C-reactive protein and/or procalcitonin levels.
Culture and laboratory findings
N. meningitidis is an aerobic, oxidase-positive, gram-negative diplococcus (coffee bean shape) that grows best at 35°C to 37°C (95°F to 98.6°F) in a moist environment of 5% to 7% carbon dioxide (candle jar). The gold standard for diagnosis of systemic meningococcal infection is the isolation of N. meningitidis by culture from a usually sterile body fluid such as blood and/or cerebrospinal fluid (CSF) (most common), or synovial, pleural, or pericardial fluid. For culture of a normally sterile site, nonselective culture media are standard but a selective antibiotic-containing culture medium, such as Thayer–Martin, Martin–Lewis, or New York City culture medium, is necessary to reduce the overgrowth of commensals when the culture specimen is obtained from a nonsterile site such as the oropharynx, urethra, or rectum.
In meningococcal meningitis, the frequency of positive blood cultures is 50% to 60% and the frequency of positive CSF cultures is 80% to 90%. Isolation of the organism by culture not only confirms the etiology, but also allows antibiotic susceptibility testing, which has become important in light of increased antibiotic resistance, especially to the penicillins. Gram stain and culture of a skin lesion can increase the diagnostic yield, although a negative result does not exclude N. meningitidis. As mentioned earlier a number of rapid diagnostic tests (RDTs) are available ranging from PCR to simple dipstick and agglutination tests.
Chemistry and cytologic findings suggestive of bacterial meningitis include a CSF glucose concentration below 45 mg/dL (2.5 mmol/L), a protein concentration above 500 mg/dL, and a white cell count above 1000/µL. The CSF is generally cloudy with a leukocytosis consisting predominantly of polymorphonuclear neutrophils associated with hypoglycorrhachia. However, one or more of the classic findings is often absent in meningococcal meningitis. The Gram stain of the CSF is positive in about 75% of cases (Figure 143.4).
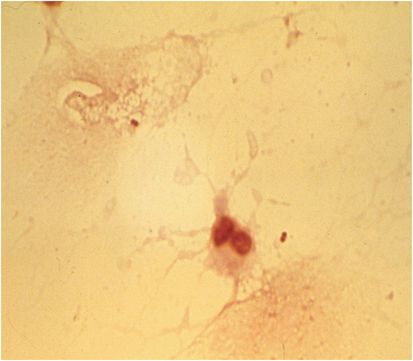
Figure 143.4 Gram stain of CSF: note gram-negative diplococci and polymorphonuclear leukocyte.
Nonspecific helpful laboratory abnormalities include an elevated serum white blood cell count, C-reactive protein, and/or procalcitonin levels.
N. meningitidis is serogrouped based on the distinct chemical composition of its polysaccharide capsule. The meningococcus has been classified as serogroups A, B, C, D, 29E, H, I, J, K, L, W (W-135), X, Y, Z, and nontypeable (indicates organism is nonencapsulated). Serogroups A, B, C, W, and Y are responsible for the vast majority of cases of invasive disease. With rare exceptions, invasive meningococci are encapsulated, attesting to the virulence conveyed by the polysaccharide capsule. In contrast, meningococci colonizing mucous membranes are usually not encapsulated.
Commercial latex agglutination kits, which can also be used on body fluids such as CSF and urine, utilize latex beads coated with antibodies to meningococcal capsular antigens to detect five capsular types: A, B, C, Y, and W, but the sensitivity for serogroup B is relatively low.
A number of rapid diagnostic tests (RDTs) are available ranging from PCR to simple dipstick and agglutination tests. Advantages of these tests over culture are rapidity, reliability in the setting of concomitant antibiotics, and in the case of PCR, simultaneous testing for infection due to N. meningitidis, Streptococcus pneumoniae, Haemophilus influenzae, or other microorganisms.
Therapy
Ceftriaxone is the drug of choice for meningococcal disease. Ceftriaxone therapy should be administered within 30 minutes of considering the diagnosis of meningococcal meningitis and is administered intravenously (IV), at least initially. Infected persons should receive 2 g IV every 12 hours for at least 7 days. Those who are β-lactam allergic should receive a fluoroquinolone or chloramphenicol.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





