32 Meningiomas
It is fair to say that few procedures in surgery may be more immediately formidable than an attack upon a large tumor of the [meningioma type], and that the ultimate prognosis hinges more on the surgeon’s wide experience with the problem in all its many aspects than is true of almost any other operation that can be named.1
Much of what was written about meningiomas by Harvey Cushing and Louise Eisenhardt in 1938 still applies today. However, there have been many recent advances in the understanding of the biology and molecular genetics of these tumors. New developments in imaging, endovascular treatments, and surgical and radiotherapy techniques have significantly improved treatment outcomes for meningioma patients. Although more technically sophisticated, our surgical approaches have become less aggressive over recent years for all meningiomas, given recent findings of no significant difference in recurrence-free survival in patients undergoing less versus more aggressive resections, as determined by the Simpson grading system.2 This is particularly true for tumors in certain high-risk locations, such as the cavernous sinus3 or those involving major venous sinuses,4 for which we now favor combined approaches of surgery and adjuvant radiotherapy, providing good tumor control with excellent functional outcomes.
 Epidemiology
Epidemiology
The recent report of the Central Brain Tumor Registry of the United States, reviewing primary brain tumor statistics from 49 population-based cancer registries, indicates that the most frequently reported histology is meningioma, accounting for 35.5% of all tumors, followed by glioblastoma (15.8%).5 By comparison with other benign histologies, meningiomas appear to be the predominant tissue type, with pituitary tumors accounting for only 14.1% and benign nerve sheath tumors 8.3% of all primary brain tumors. Benign intracranial meningiomas are more prevalent in women, but atypical and anaplastic forms appear more commonly in men. Meningiomas account for about 38% of all intracranial tumors in women and 20% in men. Generally their incidence increases with age, ranging from a low of 0.3 per 100,000 in childhood to a high of 8.4 per 100,000 in the elderly population.6 In childhood, meningiomas account for only 1 to 4% of all brain tumors and there is no female predominance. In most surgical series, the predominant tumor locations are convexity, falx/parasagittal, sphenoid wing, and skull base locations (Table 32.1).
 Classification
Classification
In 1922, Harvey Cushing coined the term meningioma to describe a benign globoid tumor arising from the leptomeninges. Since that time, various pathological classification systems have used different morphological features, proliferation indices, and pathological grading systems. The current World Health Organization (WHO) system groups meningiomas by likelihood of recurrence into three grades (Table 32.2).7,8 Meningiomas with a low risk of recurrence and nonaggressive growth are classified as grade I, whereas those with a higher likelihood of recurrence and more aggressive behavior are classified as either grade II or grade III meningiomas. Generally, grade I meningiomas are referred to as benign, grade II as atypical, and grade III as malignant.
Beyond the histology classification, the degree of meningioma resection is characterized by the system described by Simpson in 1957 (Table 32.3).9 This system takes into account the extent of tumor resection and removal of involved dura, bone, and venous sinuses. Traditionally, many studies have found that higher Simpson grade resections, when tumor is residing within dura or bone, are associated with increased risk of tumor recurrence.10,11 More recent studies, however, suggest that recurrence-free survival is not statistically different between patients undergoing Simpson grade I, II, III, or IV resections.2 Despite this, the Simpson grade of surgical resection should be part of the routine reporting of surgical results, as it reflects more subtle involvement of the dura, arachnoid, arteries, veins, and nerves that only the surgeon can observe at open operation and that is not always obvious on routine postoperative magnetic resonance imaging (MRI).
Pearl
• Adding the Simpson grade of resection and labeling index to pathological grading improves the comparability of results for different methods of treatment of meningioma.
Table 32.1 Meningioma Locations: UCSF Surgical Series 1992–2005
| Location | Number | Percent |
| Convexity | 246 | 30 |
| Falx/parasagittal | 227 | 27 |
| Sphenoid wing | 126 | 15 |
| Tentorium | 53 | 6 |
| Cerebellopontine angle | 50 | 6 |
| Olfactory groove | 44 | 5 |
| Multifocal | 30 | 4 |
| Suprasellar | 22 | 3 |
| Intraventricular | 15 | 2 |
| Foramen magnum | 13 | 2 |
| Pineal | 3 | <1 |
| Total | 829 | 100 |
Immunohistochemical markers of proliferation potential such as Ki-67, MIB-1, and proliferating cell nuclear antigen (PCNA) indices have also been correlated with risk of recurrence.12–17 Ideally, pathological grade and histology, Simpson surgical grade, and a marker of proliferation index should be included in the reporting/analysis of operative meningioma specimens (Table 32.4).
 Molecular Biology
Molecular Biology
Chromosomal and Genetic Abnormalities
It has long been recognized that the arachnoid cap cell is the cell of origin of meningiomas. Early karyotypic analysis of meningioma cells revealed abnormalities of the long arm of chromosome 22 as a characteristic feature. However, over the years, much more has been learned about the chromosomal and genetic aberrations underlying meningiomas. Progression from benign to atypical meningioma is associated with losses on chromosome 1p, 6q, 10, 14q, and 18q, and gains on 1p, 9q, 12q, 15q, and 20.18–20 The progression from an atypical to a malignant meningioma has been associated with losses on 9p and 17q.18 More recent gene expression array studies identified nine genes that are overexpressed (TPX2, RRM2, TOP2A, PI3, BIRC5, CDC2, NUSAP1, DLG7, SOX11) and two that are underexpressed (TIMP3, KCNMA1) in grade III versus grade I meningiomas.21
Table 32.2 World Health Organization Classification of Meningiomas
| Meningiomas with Low Risk of Recurrence/Aggressive Behavior | |
| Meningothelial meningioma | WHO grade I |
| Fibrous (fibroblastic) meningioma | WHO grade I |
| Transitional (mixed) meningioma | WHO grade I |
| Psammomatous meningioma | WHO grade I |
| Angiomatous meningioma | WHO grade I |
| Microcystic meningioma | WHO grade I |
| Secretory meningioma | WHO grade I |
| Lymphoplasmacyte-rich meningioma | WHO grade I |
| Metaplastic meningioma | WHO grade I |
| Meningiomas with Higher Risk of Growth/Aggressive Behavior | |
| Atypical meningioma | WHO grade II |
| Clear cell meningioma | WHO grade II |
| Chordoid meningioma | WHO grade II |
| Brain invasive meningioma | WHO grade II |
| Rhabdoid meningioma | WHO grade III |
| Papillary meningioma | WHO grade III |
| Anaplastic (malignant) meningioma | WHO grade III |
The loss of the long arm of chromosome 22 occurs in 40 to 70% of meningiomas and is associated with loss of the tumor suppressor gene for neurofibromatosis type 2 (NF2), located at 22q12.22–25 The product of this gene, merlin, is thought to be critical for meningioma tumorigenesis. Merlin belongs to the family of structural proteins that link the cytoskeleton to several proteins of the cytoplasmic membrane, and some authors have suggested that merlin may act as a tumor suppressor via its interactions with the cellular cytoskeleton.26 Recent genomic analysis of non-NF2 meningiomas reveals mutations in TRAF7 (a proapoptotic E3 ubiquitin ligase), KL4 (a transcription factor involved in inducing pluripotency), AKT1 (a phosphatidylinositol 3-kinase [PI3K] activator), and SMO (a Hedgehog signaling activator).27 These non-NF2 meningiomas with their unique genomic profile are also clinically distinctive. They are nearly always benign, have chromosomal stability, and originate from the medial skull base. In contrast, NF2 meningiomas are more likely to be atypical, have genomic instability, and arise from the cerebral or cerebellar hemispheres.27
Table 32.4 Recommended Reporting of Surgical Cases
| Index | Option |
| Pathological grade | WHO (2007) |
| Extent of surgical resection | Simpson grade |
| Marker of proliferation potential | MIB-1, PCNA, Ki-67 |
Cell Surface Receptors
Meningiomas express a variety of nuclear and cell surface receptors.6 Somatostatin, dopamine, epidermal growth factor (EGF), platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), insulin-like growth factor (IGF), transforming growth factor (TGF), basic fibroblast growth factor (bFGF), and endothelin-1 have all been found in meningiomas, although their role as therapeutic targets is still under investigation. Bevacizumab, an anti-VEGF antibody, demonstrated only modest activity against meningiomas in two recent reports.28,29 Several other PDGF-receptor inhibitors (tandutinib, dasatinib, nilotinib, sunitinib, pazopanib, and CHIR 265) are currently being studied as potential therapeutic targets for meningiomas.30
Sex steroid hormone receptors such as progesterone, androgens, and estrogen, have long been linked to meningiomas, although their significance in relation to tumor development and growth remains unclear.6,31 Most clinical studies demonstrate the female predominance for intracranial meningiomas (2:1), yet the majority of meningiomas are estrogen receptor negative. There are many reports of meningiomas changing in size with menstrual cycle phase, menopausal status, and pregnancy.31,32 Women on hormone replacement therapy, which is primarily estrogen-based, are frequently taken off their medication if a meningioma is incidentally discovered. However, there is no convincing evidence of a causal relationship between hormone replacement therapy and meningioma development or growth.33 The progesterone receptor is active in normal meninges but is also strongly expressed in benign meningiomas, suggesting a role in tumorigenesis. However, it is significantly reduced or absent in higher grade tumors, and prior attempts to control tumor growth of recurrent benign and malignant meningiomas with antiprogesterone agents have been unsuccessful.
Controversy
• The role of estrogen and progesterone in the development and growth of meningiomas remains to be clarified. Until then, the use of oral contraceptives and hormone replacement therapy in patients with a known meningioma should be considered on a case-by-case basis.
 Imaging Studies
Imaging Studies
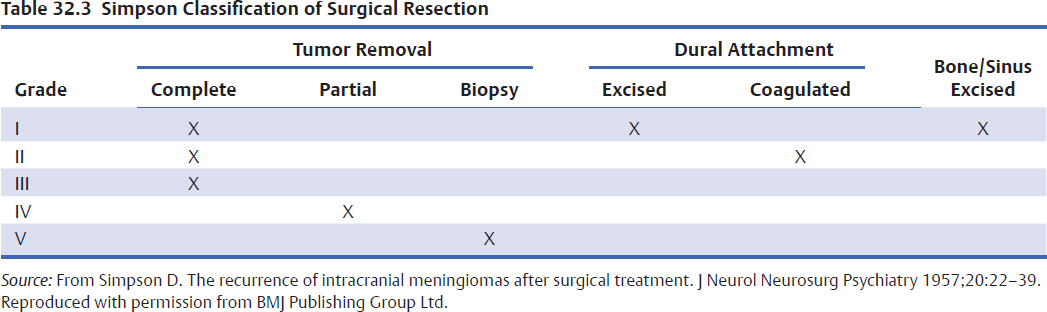
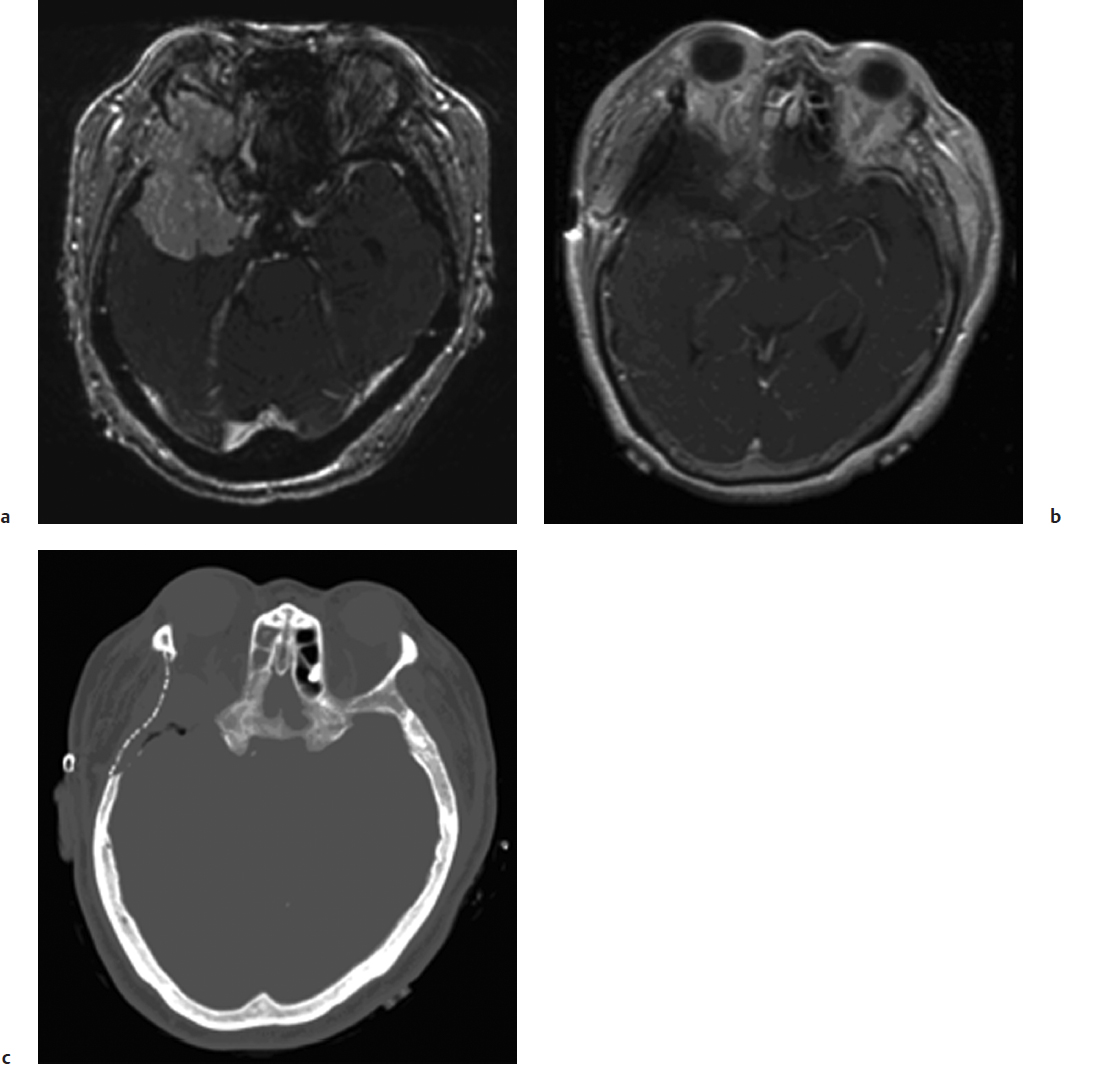
Meningiomas are often discovered incidentally when patients are worked up for headaches. Most meningiomas are isodense to surrounding brain but may have microcalcifications that are seen much more clearly on computed tomography (CT) than on MRI (Fig. 32.1). Psammomatous meningiomas can have particularly striking calcifications. CT imaging also provides better definition of bony involvement such as hyper-ostosis, which contains meningothelial cells within haversian canals at the histological level. The extent of bony involvement on pre- and postoperative CT imaging is important for determining the extent of surgical removal and the risk of recurrence. CT can also be used with image-guided surgical systems particularly for the excision of hyperostotic skull base meningiomas. Following the administration of intravenous contrast agents, these tumors show intense enhancement on CT. Their margins are usually smooth, and the enhancement pattern homogeneous. Surrounding vasogenic edema is often not well visualized on CT and can be better seen on MRI sequences.
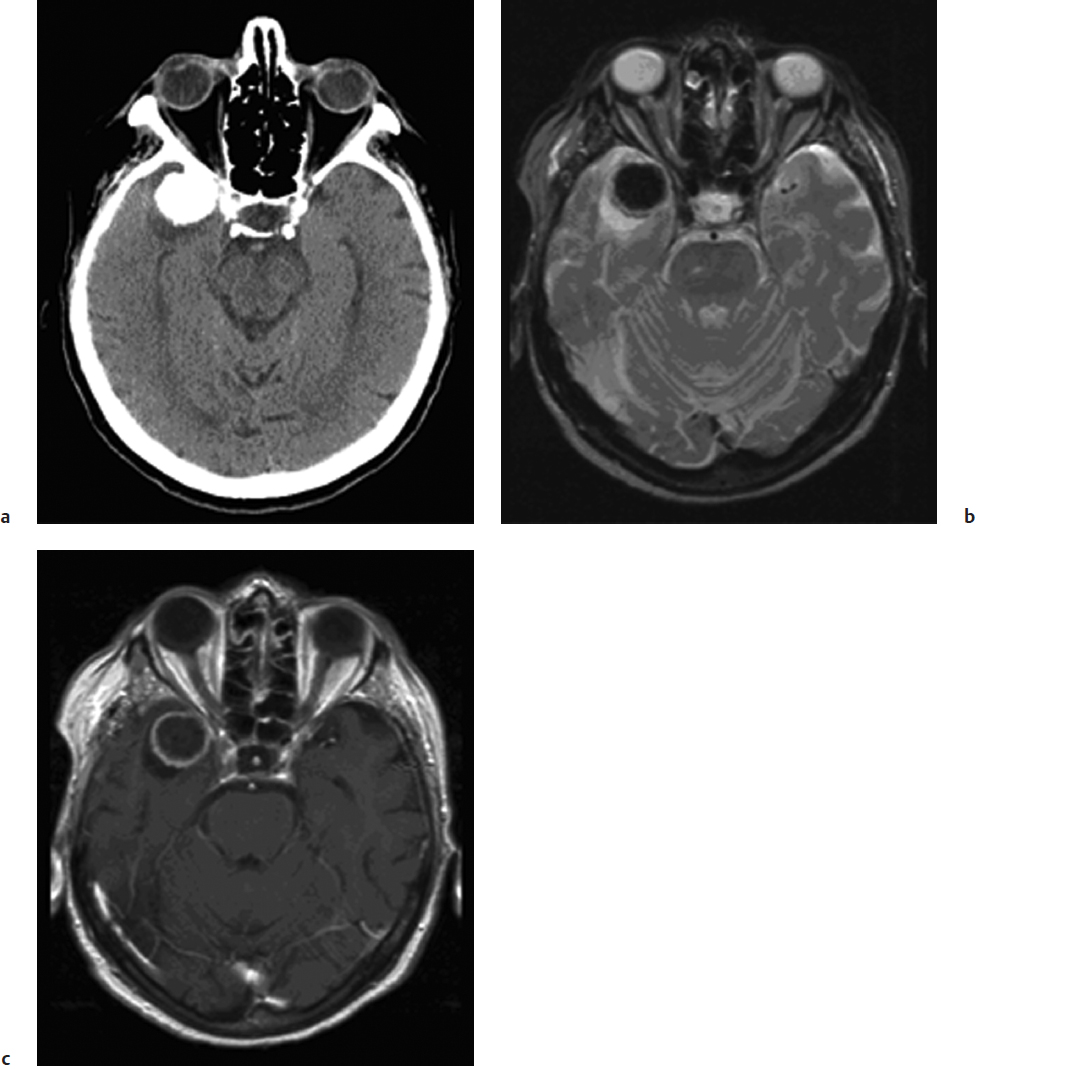
Magnetic resonance imaging is the current gold standard for imaging of intracranial meningiomas. These tumors are usually isointense on T1-weighted images and have variable signal characteristics on T2-weighted images. Following the administration of gadolinium contrast, there is intense enhancement, which may persist for several hours after the imaging studies. Many globular meningiomas show a characteristic dural tail, which is thought to represent a hypervascularity in the dura immediately adjacent to the tumor base.34 Features such as heterogeneous enhancement in the absence of embolization, irregular borders, mushrooming, regional multifocality, and abundant surrounding vasogenic edema may be imaging signs of aggressive histopathological meningioma types and clinical behavior (Fig. 32.2). The presence of edema usually also indicates for the surgeon that the arachnoid plane will be obliterated by the pial blood supply, making the dissection more tedious and less discrete.
Metabolic information related to in vivo imaging studies is currently evolving, whereas magnetic resonance (MR) spectroscopy has not yet been widely applied for meningiomas. However, MR perfusion studies may help distinguish between meningiomas and durally based hemangiopericytomas or schwannomas.35,36 A recent application of MR perfusion imaging involves intra-arterial injection of dilute contrast media into selective intracranial vessels by interventional radiologists, followed by perfusion-weighted dynamic-susceptibility contrast MRI. This enables good assessment of tissue fed by specific vessels before and after embolization treatment, which is often used as a preoperative adjuvant therapy to mitigate surgical blood loss.37
Cerebral angiography is sometimes used to assess the arterial supply of larger meningiomas, the status of major blood vessels displaced or narrowed by basal meningiomas, and the patency of large venous sinuses in the case of convexity, parasagittal, peritorcular, or tentorial meningiomas. For large convexity meningiomas, preoperative embolization can lead to reduced blood loss, shorter hospital stays, and decreased operating times.38 Studies have also shown that angiography complication rates are low, approximately 2.5%.39 However, some surgeons have questioned whether preoperative embolization for certain tumor locations offers any advantage. The decision to embolize preoperatively should therefore be made on a case-by-case basis.
Pitfall
• For large meningiomas and those with surrounding edema, there may not be a clear arachnoid plane that separates tumor from brain. The resulting operative dissection may be subpial and associated with new transient or permanent neurologic deficits.
 Treatment
Treatment
Selection of Treatment Method
Not all patients with intracranial meningiomas require surgery. The decision to operate should take into account both patient- and tumor-specific factors, as well as surgical risk/benefit ratios. The surgeon should decide whether the meningioma seen on imaging studies correlates with the patient’s symptoms and signs. If the patient has no symptoms or signs consistent with the meningioma seen on imaging, then a period of observation is recommended. In patients with no underlying medical conditions, interval 6-month MRI scans for 1 year are reasonable. If there is more than 2 mm of growth in one dimension in 1 year, then a decision regarding treatment is warranted. If there is no documented growth by 1 year, then annual interval scans should be used to help determine the biological behavior.
The neurosurgeon should consider a patient’s age, expected survival based on life table analyses, performance status, and neurologic condition when deciding to operate. The surgical risks are affected by associated medical conditions such as hypertension, diabetes, coronary artery disease, and prior cerebral vascular disease. Tumor factors such as size, location, vascularity, and involvement of sinuses must also be considered in determining the extent of resection that is possible. In the past 20 years, refined skull base techniques were developed for approaching tumors within the cavernous sinus; but over time, experience has indicated that many of these tumors can be treated with less aggressive surgery and adjuvant therapies to preserve good quality of life. In those patients who are symptomatic from their meningioma or in whom tumor growth is documented, surgery remains the primary form of treatment, but stereotactic radiation or radiosurgery is also an excellent option for smaller tumors.
Special Consideration
• Incidental, asymptomatic meningiomas can be safely monitored with regular imaging, thus deferring or avoiding surgery.
• T1-weighted post–gadolinium contrast imaging should not be used with perfusion sequences to visualize meningiomas, as both tumor and arteries will appear white.
Surgical patients require preoperative evaluation of medical conditions and often need preoperative steroids to optimize surgical conditions. Many surgeons also prescribe perioperative antiepileptics for these patients. To date, there are no class 1 data to indicate that seizure prophylaxis provides long-term reduction in seizures. However, surgery for meningiomas is frequently associated with perioperative seizures, particularly in certain locations, so perioperative antiepileptics may be helpful in reducing them. Frontal, temporal, and parietal locations have a higher incidence of associated seizures than occipital locations, and parasagittal/falx locations are at greater risk for seizures than basal or convexity locations.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree